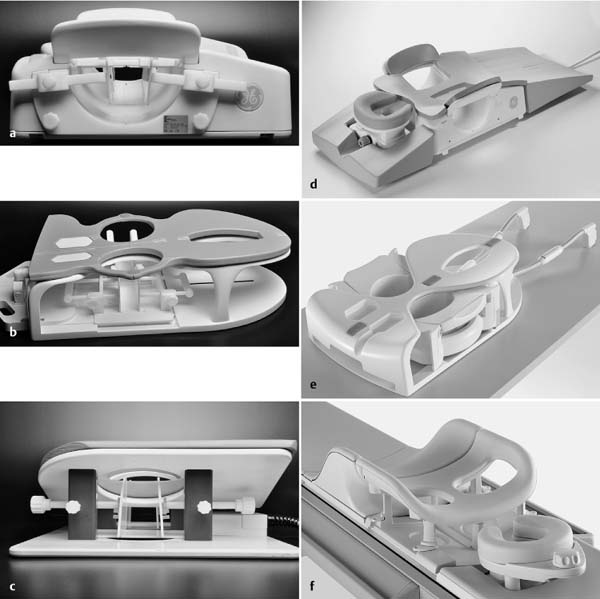
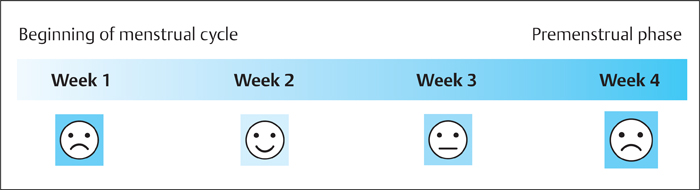
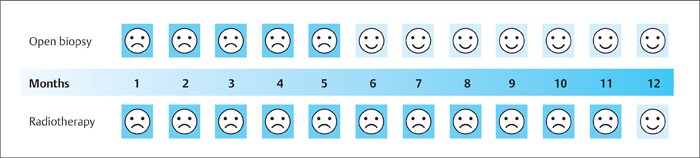
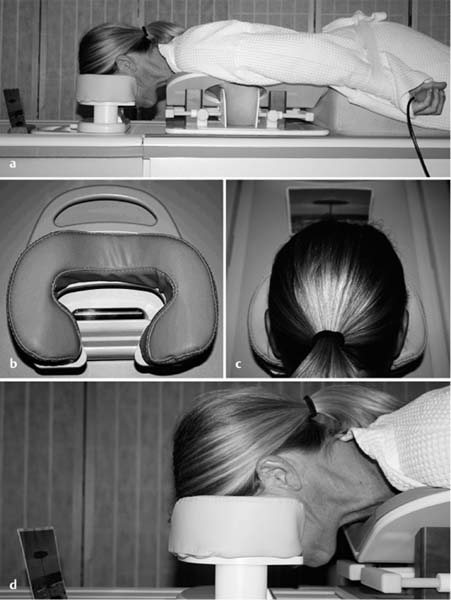
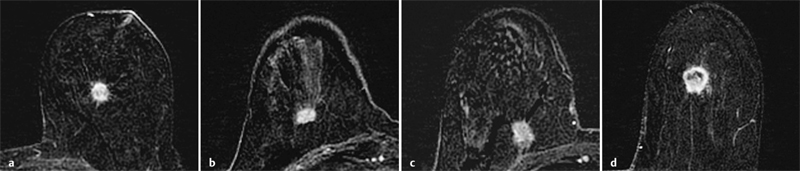
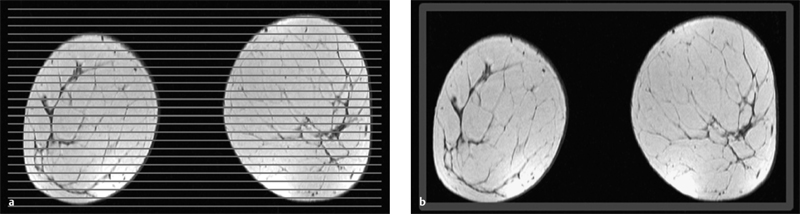
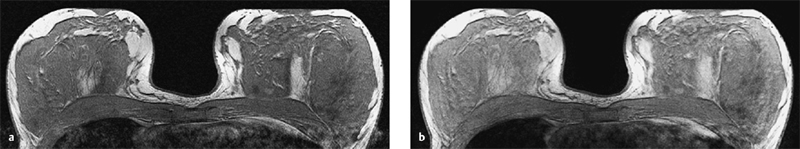
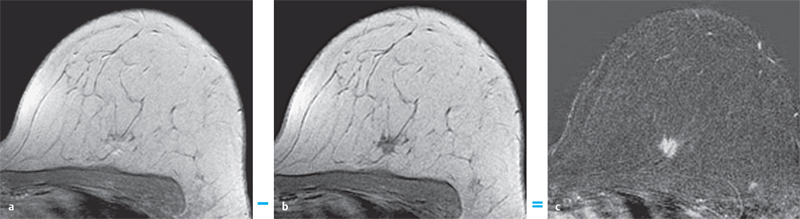
3 Technique and Methods Since Paul Lauterbur published his first reports on magnetic resonance imaging (MRI) 30 years ago, it has developed into the mature and versatile imaging technique of today. The method employs the physical principles of nuclear magnetic resonance (NMR), which have been described by several chemists and physicists including Nobel prize winners Isidor Rabi, Edgar Purcell, Felix Bloch, and Richard Ernst. According to the laws of electromagnetism, the rotating motion of an electrically charged particle induces a localized magnetic field around the particle. Certain nuclei, namely the proton and those possessing an uneven number of protons and neutrons, have an intrinsic angular momentum or spin. For MR imaging, the hydrogen nucleus, or proton, is preferred because of its high concentration in biological tissues (water, fat). In addition to its high abundance, it has the highest magnetic moment of all natural isotopes. In the absence of an external magnetic field, the orientations of the magnetic spins are at random, so that their magnetic dipoles have no net external effect. When hydrogen nuclei are placed in a strong external magnetic field, these atomic nuclei align themselves with the static magnetic field B0 in one of two orientations (magnetic quantum numbers): parallel, i.e., spin up (low-energy state), or antiparallel, i.e., spin down (high-energy state). Because the energy difference between the two states is small, they are almost equally probable. For protons at 1.5 T, the lower energy state is favored by only 1 additional proton per 100 000 protons. In addition, each proton is spinning around its own axis and experiences a torque when exposed to a magnetic field, similar to a spinning top in the earth’s gravitational field. The spin vectors are forced to precess or gyrate at a certain frequency, which is linearly dependent on the magnetic field strength B0. This precessional frequency ù of the proton’s magnetic moments, also called the Larmor frequency, obeys the formula: The proportionality constant γ is specific for the type of nucleus and is called the gyromagnetic ratio. The joint alignment of the spin vectors in the magnetic field creates the macroscopic magnetic moment or magnetization M. It is this net magnetic moment that is responsible for the induction of the MR signal in the receiver coil. Excitation and relaxation. A short burst of radiofrequency (RF) excitation at the appropriate material-dependent resonant frequency (42 MHz for protons at 1 T) causes a reorientation of the proton’s magnetization vector out of its alignment with the longitudinal axis of the magnetic field; that is, the macroscopic magnetization experiences the torque of the RF field and is forced to rotate about it. The degree of displacement, the flip angle á, is dependent on the amplitude and duration of the excitation pulse and is expressed in angular degrees. After the RF pulse is turned off, the protons resume their original positions of longitudinal alignment with the static magnetic field, i.e., they relax. During this relaxation process, the energy absorbed by the nuclei is emitted as RF radiation, or lost through molecular interactions such as electromagnetic dipole–dipole interactions and thermal dissipation. The emitted electromagnetic signal (MR signal) can be detected as an induced voltage by special receiver coils and translated into an image. The restoration of the magnetization vector to its original orientation is an exponential process described by the increase of magnetization in the longitudinal plane (spin–lattice interaction associated with the T1 relaxation time), and the loss of transverse magnetization (spin–spin interaction associated with the T2 relaxation time). The decay of transverse magnetization is caused by fluctuations of the molecular dipole fields that cause dephasing of the precessing spin vectors. Field inhomogeneities give rise to an additional dephasing of the aligned spins, denoted by adding a star to the relaxation time T2 (T2*). The relaxation times are the times it takes for the RF signal to exponentially rise (T1) or decay (T2) to half its maximum value. Fortunately for the contrast in MR images, T1 and T2 relaxation times are unique for each type of tissue. They can be measured by the receiver coil and are used for image generation. Spatial encoding. For image generation, the emitted signals or RF echoes must be assigned to a specific location on a three-dimensional matrix. Spatial localization is achieved by applying linear magnetic field gradients in x, y, and z directions in space. Twodimensional (2D) and three-dimensional (3D) techniques differ in the process of spatial encoding. In the 2D technique, section selection is achieved by simultaneously switching the slice-selection gradient and RF excitation, followed by a phase and frequency encoding in-plane. In the 3D technique, a volume is excited with RF and a second phase encoding is performed orthogonally to the first. Contrast. The contrast of MR images is influenced by two factors: tissue-specific factors and external factors. Major tissue-specific factors include proton density, and T1 and T2 relaxation times. External factors include hardware and software parameters (e.g., slice thickness, orientation, number of acquisitions), the selected pulse sequence (e.g., spin-echo, gradient-echo, fat saturation), the field strength, and whether or not contrast material is administered. Pulse sequences. There are numerous different pulse sequences that can be used for acquiring MR images. Some basic aspects of spin-echo (SE) and gradient-echo (GE) sequences, the latter of which is especially important for dynamic MR mammography, are explained in the following text. The echo in GE sequences is not produced by a 180° RF pulse but by reversing the gradient, causing a refocused RF echo, i.e., gradient- echo. In addition, the initial 90° RF pulse can be substituted by a smaller pulse with a flip angle α < 90°, which does not use the entire longitudinal magnetization. Although this results in a decreased signal intensity compared with that of the SE sequences, image generation and acquisition are much faster. Due to the greater sensitivity of GE sequences to local field inhomogeneities caused by ferromagnetic substances (e.g., metal clips) and to the susceptibility differences of adjacent tissues (e.g., bone/air, hematoma/normal tissue interfaces), they are more prone to artifacts than SE sequences. The echo time length, however, is also a factor with a major influence on artifact formation. The greater sensitivity of GE sequences to paramagnetic contrast materials is an additional consequence of these factors. Contrast material. MRI signal production can be modified by the administration of contrast material. Paramagnetic substances are primarily used for this purpose, but superparamagnetic materials are also administered. Both groups of materials have the property of changing the relaxation times of the imaged anatomical structures. The major effect of paramagnetic contrast materials is to shorten the T1 and T2 relaxation times of the tissues. Dynamic MR mammography takes advantage of this shortening effect on the T1 relaxation time, resulting in signal enhancement in the T1- weighted sequences. Superparamagnetic substances, which exert a strong T2 shortening effect, are not utilized in MR mammography. To achieve adequate spatial resolution in MR mammography, dedicated surface coils made especially to fit the breast shape must be used. Commercially available devices that allow a simultaneous bilateral examination are designed for the patient lying prone with both breasts hanging freely in the lumen of the breast coil. Although unilateral breast coils have the advantage of a more homogeneous magnetic field, they have the disadvantage of requiring the patient to return for a second examination on another day, and are therefore no longer employed. Currently only bilateral breast coils are used, allowing the simultaneous MR examination of both breasts after a single administration of contrast medium (CM). It is of great advantage for the surface coil to be open to the lateral sides, allowing access to the breast after positioning of the patient. In this way, it is possible to position and immobilize the breasts under visual monitoring (Fig. 3.1). It is also a prerequisite for the performance of MRI-guided interventional procedures. Fig. 3.1a–f Open breast surface coils from various manufacturers. a GE HealthCare. b Noras. c Invivo. d GE HealthCare. e Siemens. f Philips. The circulation in the female breast is subject to hormonal changes. As expected, CM uptake in the breast also varies with the phase of the menstrual cycle and the endogenous hormonal status. The degree of disturbing signal enhancement is least in the second week of the menstrual cycle, making this the preferred time slot in which to perform breast MRI if the referral indication allows. In spite of the fact that focal contrast-enhancing patterns caused by cyclic fluctuations rarely show a pattern typical for malignancy, MR mammography examination in the first and fourth weeks of the menstrual cycle should be avoided (Fig. 3.2). The problem arising from the normally increased parenchymal enhancement pattern seen at an unfavorable phase of the cycle is rather that especially the small and non–massenhancing lesions may be masked and more easily missed. In addition to the performance of an early image subtraction, it is therefore recommended to perform an earliest subtraction when MR mammography shows a strong parenchymal enhancement. If a woman has no menstrual cycle that can be used to asses her hormonal status and give her an appropriate appointment, cyclic breast pain is a reliable criterion for estimating a favorable cycle phase. MR mammography appointments should not be given to coincide with a time when breast pain is strong. Hormone replacement. There is no restriction on the optimal time for performing MRI of the breast on a postmenopausal woman. However, hormone replacement therapy (HRT) can result in undesirable early contrast enhancement of the parenchyma. Here also, the earliest subtraction should be performed to get the most information out of the examination. In exceptional cases, it may be expedient to repeat the examination 4–6 weeks after discontinuing HRT. In our experience, however, it is not routinely necessary to discontinue HRT before performing an MR mammographic examination. Fig. 3.2 Optimal appointment week for performing MR mammography in terms of the menstrual cycle. The best time to perform a breast MRI examination is during the second week of the menstrual cycle. Less optimal is the third week of the cycle. The least favorable times to perform this examination are during the first and fourth weeks of the cycle. MR mammography may be performed without limitation after both fine-needle biopsy and large-core biopsy of a breast lesion without significant hematoma. Neither of these procedures results in increased contrast enhancement and they do not, therefore, interfere with the interpretation of the examination. After carrying out a vacuum-assisted biopsy one typically finds a relatively large resection cavern with reactive hyperemia at the borders and a central hematoma. Residual tumor (e.g., seen as circumscribed enhancing areas) is occasionally seen under these conditions but is not detected reliably. Galactography. If medical and organizational reasons allow it, galactography should not be performed before a planned MR mammography examination because areas of increased enhancement may result in the examined breast segment. If galactography has already been performed, then the differential diagnosis of a segmental contrast enhancement pattern in the examined duct must include reactive hyperemia. Open biopsy and radiation therapy. To avoid problems in interpreting areas of increased enhancement due to wound healing, MR mammography should not be performed earlier than 6 months after open biopsy. After lumpectomy followed by breast irradiation, this interval is increased to 12 months after completion of radiation therapy (Fig. 3.3). However, the degree and duration of changes caused by radiation therapy are subject to significant interindividual differences: they may resolve within a few weeks in some patients, or persist for 1–2 or more years in others. Fig. 3.3 Interval between previous open biopsy or irradiation and breast MRI. It is recommended to wait 6 months after open biopsy, and 12 months after breast irradiation following breast-conserving therapy before performing MRI of the breast. Normally, the patient is examined in a whole-body scanner in the prone position with both arms lying flat against the body. If the patient is wearing a bath robe, the arms can be placed in a loop made from the knotted belt. This has the advantage of allowing the patient to relax the shoulder area (Fig. 3.4a), significantly reducing the risk of motion artifacts due to relaxation of the pectoral muscle and adjacent breast tissue during the examination. As an alternative, the arms may be placed above the head. This positioning, however, increases the risk that the breasts are not completely placed inside the breast coil since the cranial portions of the breast are pulled partially out of the coil when the arms are raised. The use of a special head rest, upon which the forehead rests and from which one can look at a picture or postcard through an inclined mirror, has proven useful for relaxing the patient (Fig. 3.4b–d). Positioning the head sideways is often found to be uncomfortable and to result in cervical complaints. After sliding the patient into the magnet bore, it is important to be assured that the extension tubing of the venous access is freely accessible. Fig. 3.4a–d Patient positioning for breast MRI. a Comfortable patient positioning in an open whole-body scanner in the prone position with both arms lying flat against the body, in the bathrobe belt loop. b Padded head rest with integrated inclined mirror. c Patient head positioning with view of a picture postcard. d Lateral view of head positioning. Adequate breast compression is necessary to effectively reduce motion artifacts during an MR mammographic examination. This is especially true for the subtraction technique generally used in Europe. Such artifacts are less disturbing in the primary fat saturation technique. There have been numerous endeavors to achieve better compression of the breast inside the coil. Wearing a T-shirt during the examination as well as lateral coil padding have proven to be ineffective, particularly because the breasts are compressed toward the thoracic wall or medially. This is also true for ventral padding of the breast inside the coil with specialized inserts in different sizes. The industry offers a coil-integrated mediolateral compression device. This leads to a maximal spread of breast tissue in the craniocaudal direction, which is undesirable when using the standard axial MRI view. In contrast, it is optimal to use open surface coils with integrated, manually guided compression paddles in the craniocaudal direction (Fig. 3.5). Aside from the resulting reduction of motion artifacts, this device decreases the breast thickness in the craniocaudal direction, thereby significantly reducing the slice thickness in the axial MRI view. Fig. 3.5a–g Breast compression in an open breast coil. a Insertable compression device for bilateral immobilization of the breast in an open surface coil (Noras). b Device inserted into an open surface coil (open position). c Device inserted into an open surface coil (closed position). d Breast positioned in the open surface coil with the compression device open. e Breast positioned in the open surface coil with applied compression. f Original image for planning further examination (without compression). g Original image for planning further examination (with compression). Presently, systems recommended for the performance of MR mammography with contrast material have a field strength of 1.5 T (1.5 tesla) or more. It is not advisable to use systems with a field strength of 0.5 T or 1.0 T. Preliminary experiences with 3.0 T systems show that these systems are suitable for breast MRI examination and may have a higher sensitivity, though to date it is not statistically significant (Fig. 3.6). Fig. 3.6a–d Breast MR images acquired with a 3 T-system. 3D breast images of four carcinomas from MRI performed with a 3 T-system. Representative single subtraction images (images courtesy of Radiology group practice, Bochum, Germany). 2D technique. For MR mammography performed using 2D technique, single axial slices are excited (Fig. 3.7a). There should be no gaps between these slices, although, for methodological reasons, there is a signal decrease in the peripheral areas of each slice which has no clinical significance. The field of view (FOV) is rectangular and also includes an area within the intrathoracic space. The repetition times (TR) lie in the range of ~200–350 milliseconds, allowing the acquisition of a sufficient number of slices within the TR interval. The ideal flip angle is between 70° and 90°. 3D technique. In 3D technique, the entire breast is excited as a volume (Fig. 3.7b). This volume can be divided into so-called partitions, or slices of variable thickness in any desired plane. 3D imaging allows the depiction of thin slices without gaps in a defined slice profile. The selected repetition times are on the order of 10 milliseconds and shorter than those for the 2D technique. The flip angle is typically 25°. Contrast-enhanced breast MRI. Nowadays both the 2D and 3D techniques may be used without reservation for contrastenhanced MRI of the breast (Fig. 3.8). Both techniques have advantages and disadvantages that do not significantly influence the assessment or predictive value of the breast MRI. The decision on which of these techniques to use depends largely upon the experience of the examiner and it is therefore not advisable to frequently vary the technique used. Fig. 3.7a, b Acquisition of breast MRI images in 2D and 3D techniques. a Image acquisition of single slices in 2D technique. b Image acquisition of a volume block in 3D technique. Fig. 3.8a, b Comparison of breast MRI images in 2D and 3D techniques. No significant difference in image quality is seen. a Single precontrast T1w slice in 2D technique. b Single precontrast T1w slice in 3D technique. The development and implementation of parallel imaging was motivated by the wish to decrease the image acquisition time by reducing the number of phase-encoding steps without decreasing the spatial resolution. Basically, parallel imaging is the acquisition of image data from two or more receiving coils with varying spatial sensitivity. Images are then reconstructed from the complementary data received from each coil element rather than using the time-consuming sequential phase-encoding steps. Techniques. There are two main groups of parallel imaging algorithms used for image reconstruction: First there is image-based reconstruction after Fourier transformation (SENSE, PILS, and SPACE RIP) by which images with overlapping anatomical coverage obtained from several surface coil elements in phased array are used to form the final image. Second there is k-space-based reconstruction before Fourier transformation (SMASH, AUTOSMASH, and GRAPPA) in which missing k-space links are calculated by summing k-space data from each coil element and filled in. T1-weighted (T1w) measurements allow the detection of tissue enhancement after administration of an appropriate paramagnetic contrast material. However, the signal-intense fat tissue can significantly reduce the probability of detecting contrastenhancing lesions in T1w sequences. This makes it essential to suppress or eliminate the fat signal in such studies. There are two basic means of achieving this: Image subtraction. In Europe, subtraction of corresponding slice images before and after contrast administration has become the established method of choice for eliminating the disturbing fat signal (Fig. 3.9). The disadvantage of this method is its susceptibility to motion artifacts. Primary fat suppression. American groups prefer primary fat saturation sequences (Fig. 3.10a,b). This technique was introduced by S. E. Harms as the so-called RODEO sequence (rotating delivery of excitation off-resonance) which combines a frequency- selective saturation of the fat signal with a GE sequence and the so-called magnetization transfer (MT). Primary fat suppression is achieved by sending a highfrequency impulse at a shift of 220 Hz just before performing the actual measurements. Saturated fat tissue then emits no signal during the examination. It is generally possible to send such a fatsuppressing impulse before any sequence, but doing so prolongs the examination time. One disadvantage of this technique is its high sensitivity to inhomogeneities of the main magnetic field. As a result it is sometimes not possible to achieve homogeneous fat signal suppression. This is particularly true for MR mammography because of the eccentric location of the breasts. In addition, breast parenchyma is signal-intense in the precontrast images when using this protocol, making subsequent image subtraction desirable (Fig. 3.10c). Fig. 3.9a–c Image subtraction to eliminate disturbing fat signal. a T1w image 3 minutes after contrast administration (second measurement). b T1w precontrast image, which is subtracted from the postcontrast image (a). c The resulting subtraction image allows optimal visualization of hypervascularized findings due to suppression of signal from surrounding fatty tissue.
Basic Principles of Magnetic Resonance Imaging
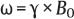
Surface Coils
 Open surface coils are one of the indicative features of highquality breast MRI equipment.
Open surface coils are one of the indicative features of highquality breast MRI equipment.
Time of Examination
Previous Breast Intervention
 It is always preferable to perform MR mammography before rather than after a breast intervention.
It is always preferable to perform MR mammography before rather than after a breast intervention.
Patient Positioning
 Patient comfort is an essential aspect of high-quality MR mammography.
Patient comfort is an essential aspect of high-quality MR mammography.
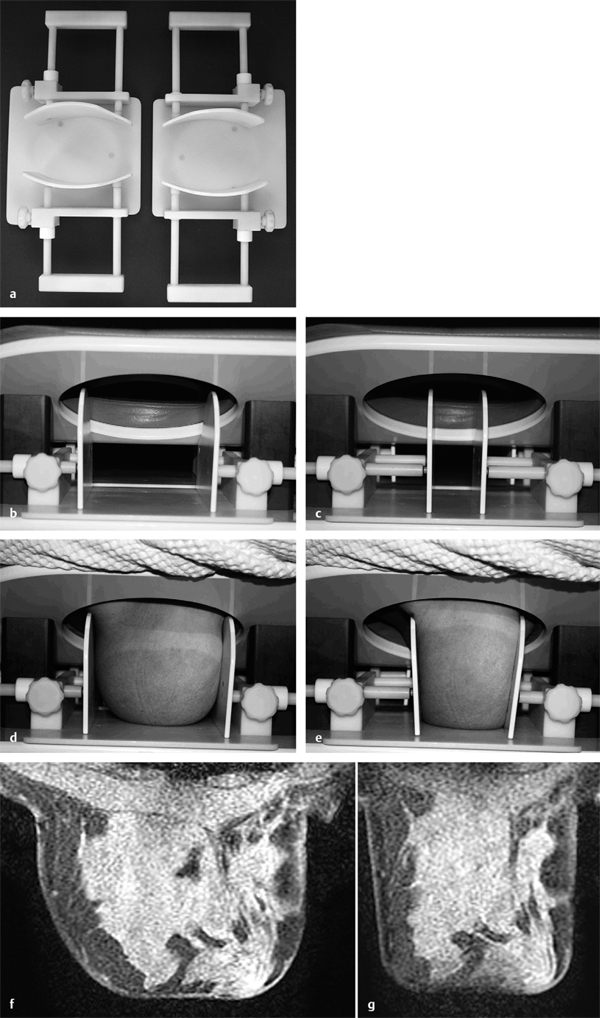
Breast Compression
 Adequate breast compression is essential for artifact-free MR mammography.
Adequate breast compression is essential for artifact-free MR mammography.
Field Strength
2D and 3D Techniques
Parallel Imaging
 Parallel imaging enables high spatial and temporal resolution in breast MRI.
Parallel imaging enables high spatial and temporal resolution in breast MRI.
T1-Weighted Sequences (with and without Fat Saturation)
 Subtraction of identical images before and after contrast administration
Subtraction of identical images before and after contrast administration
 Primary generation of fat saturation (FatSat) sequences.
Primary generation of fat saturation (FatSat) sequences.
Technique and Methods
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree