Chapter 346 Morphogenesis of the Liver and Biliary System
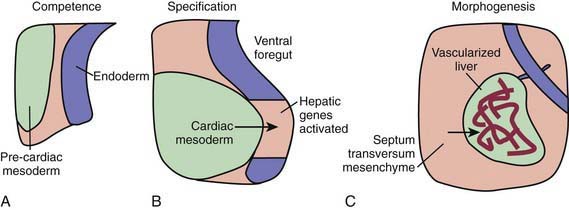
During the early embryonic process of gastrulation, the 3 embryonic germ layers (endoderm, mesoderm, ectoderm) are formed. The liver and biliary system arise from cells of the ventral foregut endoderm; their development can be divided into 3 distinct processes (Fig. 346-1). First, through unknown mechanisms, the ventral foregut endoderm acquires competence to receive signals arising from the cardiac mesoderm. These mesodermal signals, in the form of various fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs), lead to specification of cells that will form the liver and activation of liver-specific genes. During this period of hepatic fate decision, “pioneer” transcription factors, including Foxa and Gata4, bind to specific binding sites in compacted chromatin, open the local chromatin structure, and mark genes as competent. But these will only be expressed if they are correctly induced by additional transcription factors. Newly specified cells then migrate in a cranial ventral direction into the septum transversum in the 4th wk of human gestation to initiate liver morphogenesis.
The growth and development of the newly budded liver require interactions with endothelial cells. Certain proteins are important for liver development in animal models (Table 346-1). In addition to these proteins, microRNA, which consists of small noncoding, single-stranded RNA, have a functional role in the regulation of gene expression and hepatobiliary development in a zebrafish model.
Table 346-1 SELECTED GROWTH FACTORS, RECEPTORS, PROTEIN KINASES AND TRANSCRIPTION FACTORS REQUIRED FOR NORMAL LIVER DEVELOPMENT IN ANIMAL MODELS
INDUCTION OF HEPATOCYTE FATE THROUGH CARDIAC MESODERM
INDUCTION OF HEPATOCYTE FATE THROUGH SEPTUM TRANSVERSUM
Bone morphogenetic proteins 2, 4, 7
STIMULATION OF HEPATOBLAST GROWTH AND PROLIFERATION
SPECIFICATION OF HEPATOCYTE LINEAGE
SPECIFICATION OF CHOLANGIOCYTE LINEAGE
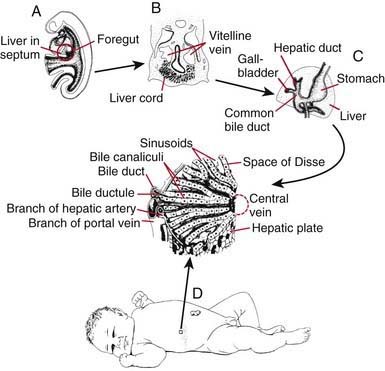
Within the ventral mesentery, proliferation of migrating cells forms anastomosing hepatic cords, with the network of primitive liver cells, sinusoids, and septal mesenchyme establishing the basic architectural pattern of liver lobule (Fig. 346-2). The solid cranial portion of the hepatic diverticulum (pars hepatis) eventually forms the hepatic parenchyma and the intrahepatic bile ducts. The hepatic lobules are identifiable in the 6th week of human gestation. The bile canalicular structures, including microvilli and junctional complexes, are specialized loci of the liver cell membrane; these appear very early in gestation, and large canaliculi bounded by several hepatocytes are seen by 6-7 wk.
The caudal part (pars cystica) of the hepatic diverticulum becomes the gallbladder, cystic duct, and common bile duct. The distal portions of the right and left hepatic ducts develop from the extrahepatic ducts, whereas the proximal portions develop from the first intrahepatic ductal plates. The extrahepatic bile ducts and the developing intrahepatic biliary tree maintain luminal continuity and patency from the beginning of organogenesis (see Fig. 346-2C).
The transport and metabolic activities of the liver are facilitated by the structural arrangement of liver cell cords, which are formed by rows of hepatocytes, separated by sinusoids that converge toward the tributaries of the hepatic vein (the central vein) located in the center of the lobule (see Fig. 346-2D
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree