Series
Year
Patients
Stomal stenosis (%)
Mitrofanoff AV
Sumfest
1993
47
19
Cain
1999
100
12
Harris
2000
50
10
Thomas
2006
67
13
Welk
2008
67
6
Malone AC
Curry
1999
300
30
Thomas
2006
50
14
Bani-Hani
2008
256
14
Rangel
2011
163
18
Most surgeons attempt to prevent stomal stenosis at the time of the creation of the channel by employing a U or V-shaped cutaneous flap to the spatulated end of the appendix. Despite these efforts as well as the continual ongoing passage of a catheter through the channel on a daily basis to gently dilate the stoma and theoretically minimize stenosis, scarring and tightness of the orifice can occur. The likely culprit is poor vascularity of the distal tip of the appendix resulting in ischemia and scarring. Sometimes hypertrophic scarring (e.g., keloids) can result in difficulty passing the catheter.
When stomal stenosis occurs the patients may report some difficulty or pain passing the catheter into the main part of the channel in mild cases but be unable to engage the catheter into the stoma at all in severe situations. This can also lead to forced attempts to place the catheter resulting in a false passage of the channel and rupture of the appendix and even loss of the conduit. In our experience, the stomal stenosis rate seems to be higher in the appendicocecostomy channel used to perform the antegrade continence enema when both channels are created concomitantly using a split appendix technique. This could be due to the fact that the ACE stoma is only accessed once a day to once every other day, as opposed to the Mitrofanoff neourethra that is accessed multiple times a day). Figure 30.1 shows two separated stomas created with this technique with the well-vascularized Mitrofanoff channel compared to the stenotic appendicocecostomy channel.
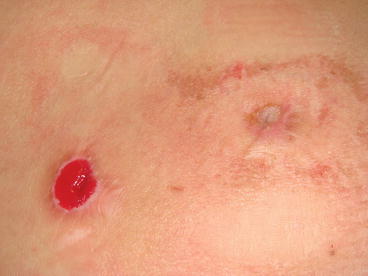

Fig. 30.1
Continent catheterizable channels in the right lower quadrant with a healthy Mitrofanoff appendicovesicostomy (left) and a stenotic Malone appendicocecostomy (right)
Initial Intervention
When patients or caregivers begin to report difficulty with catheterization at the skin level, physical examination of the stoma may reveal a contraction of the orifice or sometimes a whitish circumferential scar with no visible mucosa. Simple initial interventions include placing a warm washcloth over the stoma for a few moments to soften the orifice prior to catheterizing the stoma and avoid further trauma to the tissues. Topical steroid cream such as 1 % triamcinolone may be applied 2–3 times daily (or with each catheterization) for a period of 4 weeks (or longer if needed) to soften the cicatrix [20]. We will often give a caregiver a slightly smaller catheter to keep at home in case the usual size catheter will not pass easily. For appendicocecostomies (ACE) that are not generally cannulated but once a day or every other day for bowel irrigations, the single-use catheter used for clean intermittent catheterization can subsequently be used to gently dilate the ACE stoma 4–5 times a day after emptying the bladder.
In severe cases the stomal stenosis can be managed with intradermal injection of Kenalog 40 (1 mL = 40 mg of triamcinolone) with concomitant dilation with urethral sounds. If using this technique, it is helpful to leave an indwelling catheter in place for 72 h after the injection to allow for any inflammation to subside.
Endoscopic Management
In cases where simple topical interventions are not sufficient, inspection of the channel with a pediatric cystoscope with concomitant dilation of the channel can be employed. This can be helpful in umbilical stomas where surgical revision may be somewhat technically challenging. A guidewire can be passed into the bladder under direct cystoscopic guidance (and even manipulated out the native urethra if no bladder neck reconstruction has been performed). The cystoscope is removed and sequential dilators (Amplatz Renal Dilator Set, Cook Medical) can be passed over the wire, safely dilating the orifice. Attention should be maintained to avoid passing the rigid dilator across the continence mechanism to avoid causing iatrogenic urinary incontinence. Mid-channel strictures are less common but could be a result of an old false passage or traumatic catheterization. These can be managed in a similar fashion with careful maintenance of guidewire access. In both situations, leaving a silicone Council tip catheter (placed over the guidewire) for 1–2 weeks may facilitate healing and avoid recurrence. We have found daily gentamicin bladder irrigations to be helpful in avoiding infection with temporary catheter drainage of reconstructed bladders and catheterizable channels.
Surgical Revision
There is a relative high rate of recurrent stomal stenosis and at times, formal surgical revision becomes necessary when conservative measures have failed. A variety of techniques exist to revise a stoma at the skin surface, but in general, raising a U-shaped flap of uninvolved skin and re-spatulating the appendix through the cicatrix down to healthier tissue for anastomosis to the flap is successful. In more severe cases, the appendix can be circumferentially mobilized even down to the fascia and then the diseased portion excised. Generous double U-shaped flaps can be employed to reach the appendix for anastomosis.
Treatment Failure
In rare cases where dilation and then surgical revision have failed to prevent recurrence of stomal stenosis and major surgical revision or replacement of the channel is not ideal or desired by the patient, an indwelling tube can be employed to at least maintain the access. This would not be ideal for bladder channels but we have used them for ACE stomas in difficult cases [21]. Options include a MIC-KEY gastrostomy button (Kimberly Clark Worldwide, Inc.) or a Chait Trapdoor cecostomy catheter (Cook Medical). Both can be easily accessed to perform bowel irrigations. The former is held in with a balloon and is somewhat more protuberant from the abdominal wall. The latter features a small, soft access port that lies flat against the skin. A hinged cap opens for access to the internal part of the tube. The caregiver has to grasp and pull the tube slightly upward to open the trapdoor and engage the connector for the tubing from the enema bag. The tube is held in place by internal coils of the catheter. Both the gastrostomy button and Chait tube can be worn under clothing without any large, noticeable bulges. The gastrostomy button generally can be placed in the office setting after using a measuring device to assess the length of the channel. We have found that the Chait tube should be initially placed and subsequently exchanged in the operating room setting due to the stiffness of the coils and the need for a superstiff guidewire or rigid metal straightener. The need for exchanging the tubes is variable but in our practice is performed generally every 6–12 months or when they become heavily soiled. Complications of these chronic tubes are generally related to inadvertent displacement or granulation tissue around the catheter. The former can be managed temporarily by the caregiver at home by promptly placing and then taping in a standard catheter until a tube can be replaced electively by the provider. Granulation tissue may be treated with cauterization if symptomatic or causing troublesome bleeding.
Another alternative to stomal stenosis is to place an ACE stopper (Medicina Medical). This device is a 100 % silicone short plug with a circular 15 mm disc to keep the stopper in place and help maintain patency of the channel. They come in a variety of lengths and diameters to accommodate different size stomas (Fig. 30.2). It doesn’t pass completely through the continence mechanism so it doesn’t lead to leakage of fecal contents or erosion of the flap mechanism. The use of the stopper has been shown to decrease the rate of stomal stenosis when used prophylactically after creation of the channel [22].


Fig. 30.2
Several available sizes of the ACE stopper (Printed with permission from Medicina Medical)
Urinary Incontinence
One of the more frustrating complications of an appendicovesicostomy is urinary leakage from the stoma. This is typically reported as rare in most series ranging from 5 to 10 % [16]. However, this can be problematic for patients as the leakage is more noticeable and difficult to contain as compared to urethral leakage that may be managed with a diaper or pad. The etiology for the leakage may be technical with an inadequate or too short of a flap valve continence mechanism. Other possibilities may include underlying detrusor decompensation with disruption of the continence mechanism from elevated filling pressures. The initial evaluation should include a fluoroscopic voiding cystourethrogram as well as urodynamic testing to assess bladder anatomy, functional capacity, and bladder compliance.
Medical Management
Although an insufficient sphincter mechanism can be the culprit in channel leakage, the initial intervention, however, should be focused on maximizing the medical management. In addition, an index of suspicion for bladder deterioration or spinal cord tethering should be maintained. In a relatively small number of patients treated with an outlet procedure and a catheterizable channel without bladder augmentation, hostile bladder dynamics may result. A recent review of our experience showed that a very low outlet resistance preoperatively (in other words, a bladder never exposed to high or even normal storage pressures) and a postoperative tethered cord were independent risk factors for bladder deterioration [23]. Aggressive intervention with more frequent catheterizations, maximally tolerated doses of anticholinergics (including combinations of oral medications, intravesical preparations, and/or topical patches), and control of urinary tract infections with gentamicin irrigations are all reasonable to employ.
Botulinum A toxin injected into the detrusor muscle has been described as a treatment option in the management of high filling pressures, although the long-term durability is unknown, and thus the treatment may have to be repeated periodically. The procedure can be performed using a pediatric cystoscope and a long dextranomer-hyaluronic acid injection needle. The dosage is typically 10 IU per kg up to a maximum dose of 200 IU. In our practice, methylene blue is mixed into the solution to allow for tracking of previous injection sites.
Endoscopic Intervention
If there are no new neurological symptoms and a filling cystometrogram shows a compliant, low-pressure reservoir with a low outlet resistance, then treatments based on augmenting or revising the continence mechanism are appropriate. Initial endoscopic approaches with injection therapy can be quite helpful in these situations. With the introduction of the sterile biodegradable gel, dextranomer-hyaluronic acid, in the United States in 2001, for the injectable treatment of vesicoureteral reflux, many reconstructive surgeons have employed it “off label” for the treatment of stress urinary incontinence or fecal leakage in catheterizable channels. The procedure can be scheduled as an outpatient in the operating room under general anesthesia. It can be performed by using a 9 Fr off-set or 10 Fr all-in-one pediatric cystoscope and a long injection needle. The continence mechanism is visualized in an antegrade fashion, and the dextranomer-hyaluronic acid injection is performed circumferentially to better coapt the mucosa. Catheterization is performed after the injection prior to emergence from general anesthesia to ensure it still proceeds smoothly. The bladder can be filled and if there is leakage with gentle suprapubic pressure, more of the gel can be injected. A temporary indwelling catheter is typically not necessary. Complications are rare but can include transient difficulty catheterizing the channel as well as persistent incontinence. Bladder neck incontinence can be treated in a similar fashion through the channel combined with a retrograde approach through the native urethra. Our short-term results with injection techniques for channel incontinence have been encouraging [24]. We have also performed a suprapubic cystotomy for the sole purpose of injecting a patient’s bladder neck.
Surgical Intervention
If optimization of the anticholinergic and catheterization regimen has been performed, and endoscopic injection with a bulking agent is not successful, major surgical revision may be required. This can be approached through the original laparotomy incision. It is often helpful when revising a Mitrofanoff or ACE channel to dismember the stoma from the skin to gain more mobility. Careful attention needs to be maintained to the preservation of the vascularized pedicle. We have found that a long extravesical detrussorraphy (akin to an extravesical ureteral reimplantation) gives excellent continence outcomes along with assurance of a smooth, straight course for catheterization. It is helpful to have the bladder full when choosing the site of implantation along the sidewall of the bladder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


