Method
Type
Principle
Advantage
Reference
Cytochrome c reduction test
Direct
The reduction of ferricytochrome c to ferrocytochrome c is used to detect superoxide formation
Gold standard for measuring extracellular superoxide anions
[34]
Electron spin resonance (ESR)
Electron paramagnetic resonance (EPR)
Direct
The magnetic properties of unpaired electrons in free radicals enable them to absorb electromagnetic radiation on application of external magnetic field and this then generate absorption spectra utilizing the energy of electron spin state, which is measured by ESR spectrophotometers
Provides direct detection of the “instantaneous” presence of free radical species in a sample
Plays a major role in the assessment of most of the oxidants characterized by very short half-life (nanoseconds to microseconds) usually by using stabilizing molecules called spin-traps/probes
This is used to measure oxidative stress on proteins and lipids
Simple, high sensitivity and specificity
Detects free radicals and paramagnetic molecules. The magnetic field-based EPR detection enables nondestructive (in vitro) and noninvasive (in vivo) measurements of biological samples
EPR spectroscopy, coupled with the use of paramagnetic probes, is a potential technique for accurate and precise determination of ROS concentrations in a variety of biological samples
Xylenol orange based assay
Direct
This uses automated analyzer. The ROS in semen oxidizes ferrous to ferric ion and this forms a colored complex with xylenol orange in an acidic medium, the color intensity of which can be measured spectrophotometrically. Results are expressed in μmol H2O2 equiv./L
It is rapid, easy, stable, inexpensive, reliable and sensitive
[38]
Aromatic traps
Direct
Used to measure ROS produced in vivo. Salicylates and phenylalanine are used which reacts with free radicals to form more stable products
Used to measure ROS in cardiovascular and cerebrovascular systems
[35]
ROS measurement by chemiluminescence
Direct
Measures real time production of ROS. Uses two probes—luminol and Lucigenin. Luminol measures
Global ROS levels both extracellular and intracellular (supreroxide anion, hydrogen peroxide, hydroxyl radical)
Lucigenin is specific for superoxide anion and hydroxyl radical
Chemiluminescence is robust, sensitive and specific method
Flow cytometry
Direct
ROS measurement of hydrogen peroxide and superoxide anion by flow cytometry. Dihydroethidium measures intracellular superoxide anion and dichlorofluoroscein diacetate for intracellular hydrogen peroxide
Requires very low amounts of spermatozoa, high specificity gofer intracellular ROS in spermatozoa
Endtz test
Indirect
ROS is mainly generated by leukocytes. The myeloperoxidase is used to stain polymorphonuclear granulocytes. But does not provide any information regarding ROS generation by spermatozoa
Indirect indicator of excessive ROS generation by leukocytes in semen
Redox potential
GSH/GSSG
Indirect
Balance of reduced glutathione and its oxidized form (GSSG) gives an indication of ROS levels in vivo. GSH/GSSG levels are measured biochemically or using high performance liquid chromatography
Can be used to measure oxidative stress in-vitro and in-vivo
Total antioxidant capacity
Indirect
Measures total antioxidants in seminal plasma
Rapid colorimeter method
Thiobarbituric acid assay (TBARS)
Indirect
Measures lipid peroxidation. Detects malondialdehyde (MDA-TBA) adduct by colorimetry or fluoroscopy
Simple but non specific
Isoprostane
Indirect
Liquid chromatography-tandem mass spectrometry
Specific, stable compound
[55]
HNE-HIS Adduct ELISA
Indirect
ELISA
Rapid, helps in quantification
DNA damage
Indirect
Measures single and double stranded DNA fragmentation by sperm chromatin structure assay, TUNEL assay, sperm chromatin dispersion assay or comet assay
Measure single or double strand DNA breaks, robust, sensitive method (SCSA and TUNEL)
Oxidation reduction potential
Indirect
Measures the redox balance in a given biological system. It measures all known and unknown oxidants and antioxidants in a given sample
High sensitivity, specificity and accuracy. Can be measured both in seminal ejaculates and in seminal plasma (both fresh and frozen)
2.1.1 Types of Semen Sample Used to Detect ROS
Various types of semen samples can be used to detect ROS levels, some of which include the unprocessed seminal ejaculate, the processed semen sample by swim up and by density gradient centrifugation [20, 42]. Seminal ejaculate comprises of not only spermatozoa but also all other secretions from prostate and seminal vesicles and other accessory glands and cellular components such as round cells, leukocytes and epithelial cells. Levels of ROS are reflective of the de novo status of the ROS in the sample. In a simple wash and resuspend sample, the seminal plasma is removed but the leukocytes, round cells and debris remain in the sample. The sperm prepared by swim-up separates the actively motile sperm from the non-motile sperm and the debris. Similarly, in the density gradient separation, the spermatozoa are separated on the basis of their densities, which results in the separation of actively motile and morphologically normal sperm. The density gradient technique is used to measure ROS levels in both mature and immature spermatozoa [20, 42].
2.1.2 Measurement of ROS
2.1.2.1 Nitroblue Tetrazolium Test
Nitroblue Tetrazolium or the NBT test is based on the generation of ROS by sperm and leukocytes by using the compound Nitroblue Tetrazolium. NBT is a yellow water-soluble nitro-substituted aromatic tetrazolium compound that reacts with cellular superoxide ions to form formazan derivative that can be monitored spectrophotometrically [72]. This test is based on the principle that when heterogeneous samples such as seminal ejaculate are stained with NBT; it results in the formation of colored formazan due to reduction of NBT. This has been shown to correlate with impaired sperm function [72]. NBT is an electron acceptor that becomes reduced in the presence of free oxygen radicals to form a blue-black compound, formazan [73]. The spermatozoa containing this formazan can be also be stained histochemically and scored under microscope.
The method involves preparation of NBT solution by adding phosphate- buffered saline (PBS) with NBT powder. This is then used to stain the whole ejaculate, i.e., leukocytes and abnormal spermatozoa. The tubes are centrifuged and the pellet formed at the bottom is used to make smears. The smears are air dried and using the Wright stain, the slides are stained again and scored under microscope [73]. Leukocytes are scored as: no detectable formazan (−), scattered or few formazan granules (+), intermediate density (++), and cells filled with formazan (+++). Spermatozoa are scored as follows: formazan occupying 50 % or less of the cytoplasm (+) and more than 50 % of cytoplasm (++) [73]. NBT reaction reflects the ROS generating activity in the cytoplasm of cells, and therefore it can help determine the cellular origin of ROS in semen [74].
The cells stained with NBT are NBT positive cells and there exists a relation between the NBT+ cells and the levels of ROS in the same suspension [73]. This test helps in identifying the source of ROS whether it’s sperm or the infiltrating leukocytes in the semen. It is important to distinguish between the sources of ROS, as the clinical implications of infiltrating leukocytes are different from the pathological conditions in which sperm are themselves the source of ROS [75]. The advantages of this method include that it is readily available, inexpensive and has high sensitivity. It provides information about the differential contribution of leukocytes and abnormal spermatozoa in the production of ROS i.e., the cellular origin of ROS in the sample [73]. The major limitation of this test is that presence of other cellular reductases may also reduce NBT. Furthermore, changes in the cellular content of various oxido-reductases may also alter the rates of NBT reduction [74].
2.1.2.2 Chemiluminescence Assay
Chemiluminescence is one of the most commonly employed methods used to detect ROS in semen sample [44, 75, 76]. The reaction causes emission of light, which is measured with a luminometer. The two major types of luminometer include the photon counting luminometer and the direct current luminometer. The photon counting measures the individual photon whereas the direct counting measures the current passing through a luminometer. These are measured as photons per minute or relative light units, respectively [77].
Luminometers are also classified as single tube luminometer, which can measure only single sample, or multiple tube luminometer, which can measure multiple samples at any time, and this is the one used in research laboratories. The third one is the plate luminometer, which utilizes a 96 well plate to read multiple samples at a time [77].
The basic principle of chemiluminescence is the measurement of emitted light due to a chemical reaction occurring between chemical reagents and the ROS generated. The following equation shows two reactants A and B in presence of an excited intermediate [◊] resulting in emission of light. The decay of this excited state [◊] to a lower energy level causes light emission.
[A] + [B] → [◊] → [Products] + light
There are two major types of probes used in chemiluminescence which include Luminol and Lucigenin (Table 2.2).
Table 2.2
Major types of probes for measuring ROS by chemiluminescence
Luminol | Lucigenin |
|---|---|
1. It works through one electron oxidation | 1. This works through one electron reduction |
2. Measures both intracellular as well as extracellular ROS | 2. It measures only the extracellular ROS |
3. Hydrogen peroxide radical and oxygen radical are involved | 3. It involves measuring the superoxide anion |
The reagents used are the stock luminol probe (100 mm), the working luminol (5 mm) and the dimethylsulfoxide (DMSO) solution [78]. The procedure is performed in an indirect light. A luminometer is attached to a computer (Fig. 2.1). A total of 11 tubes are used that include 3 blank tubes which contain only the PBS, 3 negative controls which contains PBS + luminol (working solution), 2 tubes which has the patient sample and + luminol, 3 positive control which contains PBS+ hydrogen peroxide (50 μL) + luminol (Fig. 2.2). The tubes are loaded in to the luminometer (Berthold, Autolumat Plus LB 953) and a real time [lot of the ROS levels produced in each sample are visualized on the computer monitor (Fig. 2.3) and the results can be visualized and printed in an excel sheet [78].


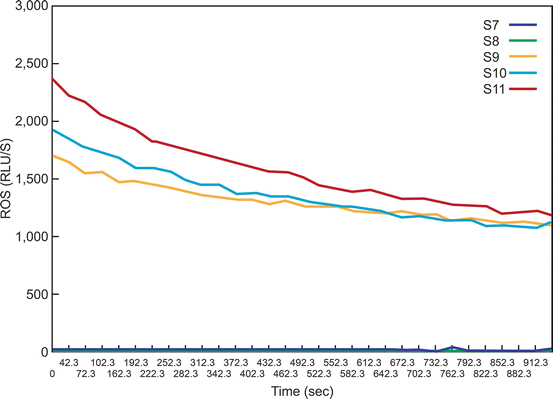

Fig. 2.1
Autolumat 953 Plus Luminometer used in the measurement of ROS by chemiluminescence assay. Multiple tubes can be loaded simultaneously for measuring ROS. The luminometer can be connected with a computer and monitor

Fig. 2.2
Preparing the tubes for ROS measurement. A total of 11 tubes are labeled from S1 to S11: Blank, negative control, test sample and positive control. Luminol is added to all tubes except the blank. Hydrogen peroxide is added only to the positive control

Fig. 2.3
A typical graph showing the ROS levels in the 11 tubes (S1–S11). As seen here, only the positive controls have significantly higher levels of ROS. Those producing low levels (Tubes S1–S8) of ROS are seen very close to the X axis
The factors affecting chemiluminescence reactions include sample volume, time of analysis, viscosity of the sample, concentration of reactants, reagent injection, temperature control, human error, background luminescence. The major advantages include that it is high specificity and sensitivity and can measure both intracellular as well as extracellular ROS [41]. The major disadvantages are: (1) it cannot measure multiple markers simultaneously and (2) the level of ROS decline with the time after ejaculation due to short half-life of ROS. Factors affecting ROS measurement are: (1) luminometer calibration; (2) sensitivity and the dynamic range as well as the units used; (3) concentration and the type of probe used; (4) concentration and the volume of semen used, and (5) temperature of the instrument at the time of measurement. Semen age, viscosity of the sample, repeated centrifugation, use of media containing albumin that can generate spurious signals; spikes and sensitivity of luminol to pH changes are other variables that influence ROS production. ROS is an independent factor of male factor infertility [26]. We have reported different cutoff values for ROS in processed semen samples [4, 6, 17] and seminal ejaculates [17, 21, 24, 43].
We have recently revised the reference range of ROS in seminal ejaculates [43]. ROS levels >102 RLU/s/106 sperm are considered abnormal. At this cutoff ROS sensitivity is 76 % with a positive predictive value of 82.1 %. When the controls were strictly comprised of individuals who had established pregnancy, the cutoff was slightly lower at <93 RLU/s/×106 sperm. The sensitivity increased to 93.8 % indicating that the test can differentiate subjects that are fertile from those that are not. Levels of ROS >102 RLU/s/×106 sperm must be considered pathological.
The major diagnostic application of chemiluminescence is that it provides independent assessment of the quality of the ejaculate and this is extremely important in patients with unexplained infertility as these patients demonstrate high levels of ROS despite conventional semen parameters within normal ranges [20, 77, 79–81]. The reproducibility of ROS by chemiluminescence assay makes the test sensitive and reliable in measuring ROS levels. ROS levels in the mature spermatozoa may have both diagnostic and prognostic importance as elevated ROS levels in mature spermatozoa may reflect oxidative stress in semen samples that will be used for ART purpose and may also be used to predict the fertilizing potential of the spermatozoa. This can be accomplished by characterizing the semen samples that are used in ART based on the established reference values of ROS.
The intracellular levels of ROS can be measured by flow cytometry using dihydrofluorescein diacetate (DCFH) to detect intracellular hydrogen peroxide radicals. This dye is oxidized to the highly fluorescent derivative dichlorofluorescein (DCF), which is detected by the use of flow cytometer [46–48, 82]. A counterstain dye for nucleic acid (propidium iodide) is used to exclude the apoptopic spermatozoa [83]. Dihydroethidium (DE) can be used to detect intracellular levels of superoxide anions [46, 48, 83]. The results are interpreted as percentage of fluorescent spermatozoa [83].
2.1.3 Measurement of DNA Fragmentation
Reduced fertility, embryo development, increased rates of miscarriages has been reported in cases of higher sperm DNA damage [84–86]. Several etiological factors such as cigarette smoking, irradiation, chemotherapy, leukocytospermia, varicocele, cancer, elevated levels of ROS, abnormalities during chromatin packaging and advancing age have demonstrated compromised sperm DNA quality [31, 83, 87–91].
Oxidative stress is responsible for single strand breaks in DNA [20, 91]. Furthermore, apoptosis can also occur as a result of increased oxidative stress and result in DNA fragmentation. Several studies show that infertile men have high number of sperm with single or double stranded fragmentation [59, 92–94].
Several tests have been introduced to measure the sperm DNA damage [64, 88, 95–101]. The methodological approaches by which sperm DNA damage is investigated in these tests are varied. Some tests measure abnormalities in sperm chromatin whereas others measure direct DNA strand fragmentation. Among such test the most commonly used are the sperm chromatin structure assay (SCSA) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) [87, 97, 99, 102, 103]. These are briefly described below:
2.1.3.1 Sperm Chromatin Structure Assay (SCSA)
Sperm Chromatin Structure Assay (SCSA) detects damaged sperm DNA using flow cytometry of acridine orange stained sperm. It is based on the susceptibility of DNA breaks to acid denaturation. Low pH treatment opens the DNA strands at the sites of breaks. Staining by acridine orange is highly precise and repeatable and comparable between fresh and frozen samples. The DNA damage is induced by exposing to denaturing conditions. This utilizes the metachromatic properties of acridine orange to distinguish single stranded/red fluorescence and double stranded/native DNA/green fluorescence [59, 60, 83]. The DNA fragmentation index (DFI) is the ratio of percentage of sperms showing red fluorescence/total fluorescence (red + green) [48, 59].
The SCSA also measures sperm with high DNA stainability (%HDS) which is related to the nuclear histones retained in immature sperm and shown to be predictive of pregnancy failure [60]. The current clinical threshold 25 % DFI that categorized patient into a statistical probability of the following: (a) longer time to natural pregnancy, (b) low odds of IUI pregnancy, (c) more miscarriages, or (d) no pregnancy [60]. The test is precise, repeatable with acceptable DNA fragmentation that has a threshold of placing a man at risk of infertility.
2.1.3.2 Terminal deoxynucleotidyl Transferees dUTP Nick End Labeling (TUNEL) Assay
TUNEL assay utilizes a template-independent DNA polymerase called terminal deoxynucleotidyl transferase (TdT) that non-preferentially adds deoxyribonucleotides to 3′ hydroxyl (OH) single- and double-stranded DNA. Deoxyuridine triphosphate (dUTP) is the substrate that is added by the TdT enzyme to the free 3′-OH break-ends of DNA (Fig. 2.4) [96, 99].
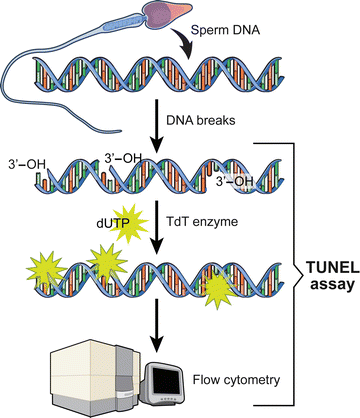

Fig. 2.4
Schematic of the DNA staining by the TUNEL assay
The more DNA strand breaks sites that are present, the more labels are incorporated within a cell. This identifies the in situ DNA breaks. Here, the 3′ hydroxyl free ends are labeled using a fluorescent label which on passing through a flow cytometer generates fluorescence, which is directly proportional to the number of strand breaks [96, 99]. Various protocols are used to detect DNA damage via TUNEL assay like fluorescein isothiocyanate labeled dUTP system and Apoptosis detection kit. DNA fragmentation can be measured by both a simple bench top flow cytometer (Fig. 2.5). This test is highly sensitive and specific [96, 99]. It measures a definite end point and is considered to provide better prediction regarding the potential of embryo implantation [86, 102].


Fig. 2.5
Set up of the bench top flow cytometer
Many factors involved in the processing, fixation and permeabilization of the specimen adversely affect the clinical implication of TUNEL assay [104]. The difficulty in the permeablization of sperm chromatin due to its highly dense compaction and tightly packed protamines plays a major role in reduced sensitivity preventing TdT from directly interacting with DNA strand breaks [105]. Unlike SCSA, the cutoff values of sperm DNA fragmentation for TUNEL have not been clearly established [61]. Studies have reported DNA fragmentation measured by TUNEL assay to range from 12 to 36.5 % at which no pregnancies were reported [106, 107]. A cutoff value of >19.2 % and 16.8 % has been recently shown to have >90 % specificity [61]. The specificities can be further increased by including only men with established pregnancies as controls. The high specificity and positive predictive value is important particularly in cases of idiopathic and unexplained infertility.
2.1.3.3 Epifluorescence Using Acridine Orange Dye
Acridine orange is a nucleic acid specific, fluorescent, cationic dye. It interacts with DNA by intercalation and by electrostatic interaction with RNA or single stranded DNA [96]. Fluorescence microscopy is used along with acridine orange dye. There is exposure to acid, which denatures DNA with single- or double stranded breaks. The dye acridine orange binds to DNA. The double stranded DNA fluoresces green and single stranded DNA gives red color. In addition the sperm suspension after staining with the Apo-direct kit for the TUNEL assay can also be visualized for DNA fragmentation by the fluorescence microscope (Fig. 2.6). Sperm stained green indicate sperm with DNA fragmentation [96]. This method uses fluorescence, which is relatively rapid, simple and inexpensive. The major disadvantage of this technique is the heterogeneous staining and color fading of the slides. Also, the presence of indistinct colors ranging from red to green interferes with the results [108].


Fig. 2.6
Fluorescent staining showing intact (red) spermatozoa and spermatozoa with DNA fragmentation (green)
2.1.3.4 Comet Assay
This is a single gel electrophoresis method, which basically measures the breaks in DNA [66]. Electrophoresis is used to mobilize DNA fragments that are produced from nucleoids after being depleted of proteins. It is based on the general concept that DNA fragments resulting from pre-existing DNA breaks have different mobility in the electrophoretic field depending on the relative size of the fragment. This generates morphological differences between nuclei containing fragmented DNA when examined under fluorescent microscopy. The resulting image represents a “comet” that consists of a head and a tail chromatin in the direction of the anode. Larger the size of the comet, higher is the level of DNA fragmentation [109, 110]. Thus, sperm with more DNA breaks shows intense comet tail [111]. At the end of electrophoresis, all the broken strands of DNA migrate towards the anode and this forms a comet tail, which can be used to assess the DNA damage using the fluorescence microscope or cytometer [64]. The comet’s tail length and fluorescent intensity is directly proportional to the degree of DNA fragmentation [65].
The assay can be performed in both neutral as well as alkaline environments. In neutral buffer, double stranded DNA damage is measured while in alkaline environment DNA can be denatured, both single– (SS) and double stranded (DS) DNA damage can be measured due to unwinding of the DNA strands [112]. Under the influence of electric field, there is separation of broken DNA strands (SS and DS) [65, 113–116]. After separation, the broken DNA fragments migrate towards the tail forming comet tail and the intact DNA remains confined to the head forming the comet’s head [117]. The major limitation of the comet assay for its routine use in fertility labs is (1) slide processing is time consuming and (2) it requires electrophoresis equipment and fluorescence microscopy.
2.1.3.5 Sperm Chromatin Dispersion (SCD) Assay
Sperm chromatin dispersion (SCD) assay uses the Halosperm kit to differentiate between non-fragmented spermatozoa from the fragmented spermatozoa [64]. This test is used in laboratories with no access to flow cytometry. It can be visualized using bright field or fluorescence microscopy. It is based on a controlled species-specific DNA denaturation to produce single-stranded DNA stretches from any DNA breaks, coupled with controlled DNA depletion [67, 101, 118, 119]. The process involves (1) integration of sperm sample into an inert agarose microgel on pretreated slide, (2) controlled acid denaturation of DNA, (3) controlled protein depletion. Normal sperm produce halos of dispersed chromatin around a dense core. In fragmented DNA, no halos of dispersed chromatin are produced [120, 121]. The halos can further be classified according to their morphology and the results can be expressed for each patient against the established cutoff criteria (Fig. 2.7). The results show a strong correlation when compared with indirect assessments of DNA damage such as the SCSA or comet assay [64, 122]. The advantages and disadvantages of the sperm DNA fragmentation assays are shown in Table 2.3.
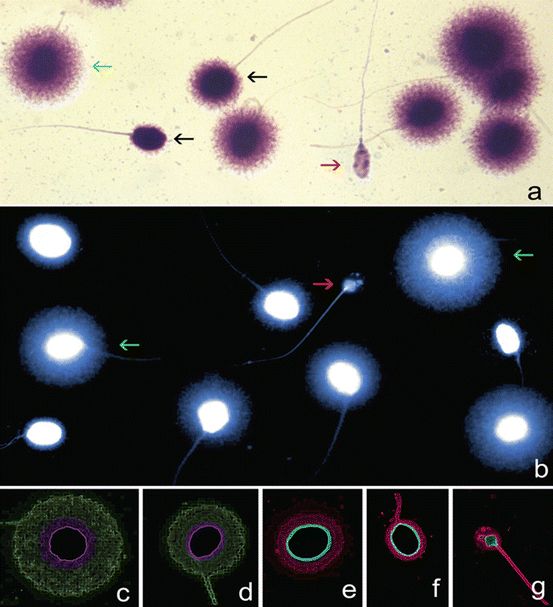

Fig. 2.7
Assessment of sperm DNA fragmentation using the sperm chromatin dispersion (SCD) test. Nucleoids from human spermatozoa obtained with the improved SCD procedure (Halosperm, Halotech DNA, SL, Madrid, Spain) under (a) bright field microscopy and Wright’s stain (b) under fluorescence microscopy and DAPI staining. Green arrows target spermatozoa containing a normal DNA molecule. Red arrows target a highly fragmented spermatozoon (degraded sperm). (c–g) Electronic filtered images showing a series of nucleoids with different levels of sperm DNA damage. Nucleoids with highlighted core delineation in green correspond to (c) large (d) and medium halos of dispersed chromatin representing a normal DNA molecule. Nucleoids in red are spermatozoa containing fragmented DNA and are represented by (e) small or (f) no halos of dispersed chromatin and (g) degraded spermatozoa. Bright-field and fluorescence microphotographs were obtained using a motorized fluorescence microscope controlled with software for automatic scanning and image digitization (Leica Microsystems, Barcelona, Spain). The microscope was equipped with a Leica EL6000 metal halide fluorescence light source and Plan-Fluotar 60 × objectives with three independent filter blocks (DAPI-5060B; FITC- 3540B and TRITC-A; Semrock, Rechestern NY, USA). A charge coupled device (Leica DFC350 FX, Leica Microsystems, Barcelona, Spain) was used for image capture (Courtesy of Prof. Jaime Gosálvez, Madrid, Spain)
Table 2.3
Assays measuring DNA fragmentation
Assay | Advantage | Disadvantage | Reference |
|---|---|---|---|
SCSA | Established clinical thresholds, robust and sensitive assay; uses metachromatic acridine orange staining and flow cytometry; requires only 10,000 cells; can be done in fresh or frozen samples | Not available in commercial kits, calculations are complex, acid induced denaturation. Not performed in routine andrology labs | |
TUNEL | High sensitivity and specificity. Can be done on fresh or frozen samples. Associated with fertility and available in commercial kits. Measures definite end point | Thresholds not standardized, not specific to oxidative damage, need for special equipment (flow cytometer or fluorescence microscope). Results affected by fixation and permeabilization of sperm. Although can be measured by fluorescence microscopy, results are subjective and prone to inaccuracy due to heterogenous nature of staining and instability of the stain | |
Comet | Requires small number of cells; high sensitivity, measures breaks in DNA; correlates with seminal parameters. Assay can be performed in neutral and in alkaline environment | Time and labor intensive, not specific to oxidative damage, requires special imaging software, lacks correlation with fertility. Requires electrophoresis and fluroscence microscopy. Slide processing is time consuming | |
SCD | Differentiates fragmented from non-fragmented spermatozoa. Does not require flow Cytometry. Can be visualized both by bright field and fluorescence microscopy Strong correlation with SCSA or Comet assay | Interobserver subjectivity to categorize the halos is a limitation of SCD |
2.1.4 Other Methods for Measuring ROS
In addition to the more popular methods of measuring DNA damage described above there are other less common techniques reported in literature. These are shown in Tables 2.4 and 2.5.
Table 2.4
Different methods to measure total antioxidants
Technique | Principle | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
TEAC | Inhibition method | Standard by other assays | [123] | |
ORAC | Inhibition method | High specificity, responds to numerous antioxidants, gross differentiation of aqueous and lipid soluble antioxidants | Time consuming | [124] |
FRAP | Reduction of Fe3 → Fe2 | Simple, inexpensive | Does not measure SH-group containing radicals | [125] |
Enhanced Chemiluminescence | Chemiluminescence | Accurate | Cumbersome, expensive instrumentation, time consuming, signal reagent might reduce in intensity | [126] |
Colorimetric | Colorimetric analysis | Less time consuming, relatively inexpensive, convenient, can be used as an in office test | Significantly expensive reagents | |
ROS-TAC Scorea | Chemiluminescence | Better predictor compared to ROS and TAC alone | Requires statistical modeling | |
ORPa using the RedoxSYS Diagnostic System | Galvanostat based mechanism | Prognostic marker (cORP), easy, less time consuming, requires less expertise, can be used for frozen specimens | Affected by viscosity of the sample |
Table 2.5
Various techniques to measure lipid peroxidation
Technique | Principle | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
Thiobarbituric acid assay (TBARS) | MDA-TBA adduct detection by colorimetry or fluoroscopy | Simple but non specific | Rigorous controls are required | |
Isoprostane | EIA/Liquid chromatography-tandem mass spectrometry | Specificity, stable compound | Labor intensive and expensive cost of equipment | [55] |
HNE-His Adduct ELISA | ELISA | Rapid, helps in quantification | Chances of cross reactivity |
2.1.4.1 Measurement of Total Antioxidant Capacity
The total antioxidant capacity (TAC) is a parameter that can be measured by evaluating the reducing ability of various antioxidants present in semen against an oxidative reagent such as hydrogen peroxide, and measuring the effect on the substrate [51, 52]. The reaction can be measured with a spectrophotometer, colorimeter, depending on the substrate. Most techniques employed to estimate TAC measure the low molecular weight, chain breaking antioxidants and do not include the contribution of antioxidant enzymes (glutathione group of enzymes, catalase, and superoxide dismutase) and metal binding proteins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


