Fig. 1
(a) AP radiograph depicting incarcerated medial epicondyle fracture in ulnohumeral joint. (b) Lateral radiograph also showing incarcerated fragment
Assessment
Clinical Presentation
Patients presenting with medial epicondyle fractures tend to be older children and younger adolescents and are generally male (Smith 1950; Chessare et al. 1977; Cheng et al. 1999). The patient may give a history of a fall onto an outstretched, extended arm and may describe an associated dislocation.
The physical examination is often variable depending on the amount of fracture displacement (Gottschalk et al. 2012). Common findings include soft tissue swelling, ecchymosis, local tenderness, and crepitus on palpation. A defect can sometimes be palpable in highly displaced fractures, although this is not always assessed for due to patient discomfort. Range of motion will often be limited secondary to pain. However, with an incarcerated fragment, there may be a block to elbow extension with a hard stop on attempted range of motion testing. Patients with an associated elbow dislocation may also have an obvious deformity.
In all patients with a medial epicondyle fracture, it is essential to test for medial elbow stability (Schwab et al. 1980; Gottschalk et al. 2012). Stress testing may be difficult secondary to pain and is generally not recommended in the alert child, but can be performed under sedation if necessary for a complete evaluation. A gravity-assisted valgus stress test may be performed (Schwab et al. 1980). This is performed by having the patient lie supine with his/her arm abducted 90°. The shoulder and arm are externally rotated 90° with the elbow held in 15 degrees of flexion (which eliminates the olecranon as a stabilizing force). Simple gravitational stress alone should demonstrate medial elbow instability. This information can be useful in guiding treatment.
Radiographic Evaluation
Radiographic evaluation of the pediatric elbow begins with the standard anteroposterior (AP) and lateral X-rays. However, radiographic interpretation of the pediatric elbow can be difficult secondary to normal variations, including eccentric ossification and multicentric ossification (Silberstein et al. 1981), as well as sex-specific variations (Patel et al. 2009). Nondisplaced medial epicondyle fractures may show a loss of soft tissue planes; however, no displacement of the elbow fat pads is usually seen, as this is an extra-articular fracture pattern. Minimally displaced fractures will demonstrate a loss of parallelism along the smooth margins of the physis.
In significantly displaced fractures, the fracture becomes quite obvious and the fragment typically remains proximal to the ulnohumeral joint line (Fig. 2). If the fragment appears at the level of the joint, it must be considered in the joint until proven otherwise (Gottschalk et al. 2012). Radiographs need to be carefully assessed for joint congruity, as medial epicondyle fractures are often associated with an elbow dislocation. Even radiographs that appear to have a congruent joint must be carefully evaluated for an incarcerated joint fragment. Previous authors have described elbows in which the joint appeared radiographically reduced despite a fragment entrapped in the joint (Haflah et al. 2010; Petratos et al. 2012; Lima et al. 2013).
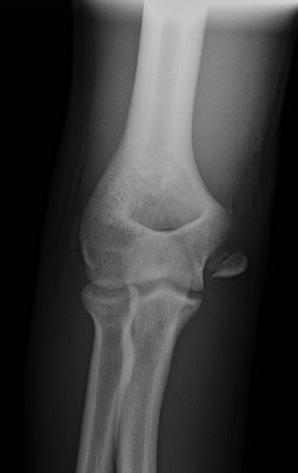

Fig. 2
AP radiograph of a highly displaced medial epicondyle fracture. Even with the high degree of displacement, it remains proximal to the elbow joint
While recognizing a medial epicondyle fracture is typically straightforward for a trained orthopedist, previous studies have demonstrated poor reliability in the measurement of displacement of medial epicondyle fractures on standard radiographs (Edmonds 2010; Pappas et al. 2010; Gottschalk et al. 2013). A 2010 study described significant variability in intra-observer agreement in measuring the displacement of medial epicondyle fractures (Pappas et al. 2010). Reviewers disagreed by greater than two millimeters 26 % of the time on AP radiographs. Disagreement occurred 64 % and 87 % of the time on lateral and internal oblique radiographs, respectively. In a study using three-dimensional computed tomography (CT) reconstructions as the gold standard, Edmonds and colleagues found that up to 1 cm of displacement was missed on both AP and lateral radiographic views, mostly in the anterior direction (Edmonds 2010). A cadaveric study in 2013, however, found excellent intra-observer reliability on internal oblique views (Gottschalk et al. 2013). The authors noted that reviewers were 60 % accurate in predicting true displacement on a 45° internal oblique radiograph and recommended multiplying the measured displacement by 1.4 to better estimate the true displacement of the fracture fragment. Other studies have also recommended the use of the internal oblique view to better estimate the true displacement of the medial epicondyle fracture fragment (Edmonds 2010).
Classification
To date, no single validated classification scheme exists for pediatric medial epicondyle fractures (Gottschalk et al. 2012; Mehlman and Howard 2012). Most classification systems describe the amount of fracture displacement and the association with an elbow dislocation. Smith provided the first classification schema in 1950 (Smith 1950). Later classification systems by Papavasiliou and Mercer-Rang followed and have been used to varying degrees in clinical practice and for research purposes (Table 1; Papavasiliou 1982; Dias et al. 1987; Lee et al. 2005; Haxhija et al. 2006). However, these classifications do not address valgus instability (Mehlman and Howard 2012). Another proposed classification schema that has been applied to medial epicondyle fractures by Gottschalk et al. is based on the chronicity of the fracture and associated displacement (Table 1; Gottschalk et al. 2012). None of these classification systems are uniformly used in guiding treatment decision-making.
Table 1
Classification of medial epicondyle fractures
Papavasiliou (1982) | |
Type 1 | Small avulsion of the epicondylar fragment |
Type 2 | Avulsed epicondylar fragment on the same level as the joint, but not incarcerated |
Type 3 | Avulsed fragment trapped in the joint |
Type 4 | Avulsion of the fragment associated with an elbow dislocation and the fragment in the joint |
Mercer-Rang (Mehlman and Howard 2012) | |
Type 1 | Minimally displaced fragment |
Type 2 | Displaced, rotated fragment |
Type 3 | Avulsed fragment trapped in the joint |
Type 4 | Avulsion of the fragment associated with an elbow dislocation and the fragment in the joint |
Gottschalk et al. (2012) | |
Type 1 | Acute |
A | Nondisplaced: physeal line remains intact |
B | Minimally displaced (<5 mm) |
C | Significantly displaced (>5 mm) |
Type 2 | Chronic |
A | Tension stress injuries |
B | Little league elbow |
Management
Treatment Controversies
There has been significant debate regarding the treatment of medial epicondyle fractures. Previous studies have attempted to summarize the existing literature, but have been unable to provide conclusive evidence to guide treatment of these fractures (Kamath et al. 2009a; Mehlman and Howard 2012). No consensus exists in the literature as to the amount of fracture displacement that warrants surgical intervention. Studies have argued for operative intervention in fractures with as little as 2 mm of displacement (Hines et al. 1987; Fowles et al. 1990; Lee et al. 2005), while other authors argue that up to 20 mm of displacement on plain radiographs is acceptable (Smith 1950; Josefsson and Danielsson 1986; Farsetti et al. 2001).
Advocates of nonsurgical management have demonstrated that patients who are treated nonoperatively have outcomes similar to or better than those who undergo surgical treatment (Smith 1950; Chessare et al. 1977; Josefsson and Danielsson 1986; Wilson et al. 1988). These authors argue patients have excellent outcomes even if the fracture heals with a fibrous union (Josefsson and Danielsson 1986).
Those who argue for surgical treatment of medial epicondyle fractures tout the importance of the medial soft tissue attachments in maintaining joint stability and allowing early elbow motion, to minimize the risk of arthrofibrosis. These authors argue that, given the mechanism of injury in medial epicondyle fractures (valgus force in extension), the capsuloligamentous and anteromedial muscular structures can be damaged and influence joint stability and eventual outcome far more than actual fragment displacement (Schwab et al. 1980; Louahem et al. 2010; Smith 1950; Kilfoyle 1965). A 2010 study of pediatric medial epicondyle fractures demonstrated that 21 % of patients treated nonoperatively developed a symptomatic nonunion requiring operative treatment (Smith et al. 2010).
The amount of fracture displacement and its exact contribution to medial elbow instability has never been clearly defined. The classic study by Josefsson suggests that the approximate rate of late valgus instability among children with medial epicondyle fractures displaced between 4 and 8 mm will be approximately 2 % (Josefsson and Danielsson 1986).
Nonoperative Treatment
Indications/Contraindications
Indications for nonoperative treatment include those patients who have a minimum amount of displacement, though current literature does not give a clear indication for the amount of acceptable displacement. Patients should also not have evidence of significant ulnar nerve dysfunction, though again this is not clearly defined. Nonoperative treatment is contraindicated in patient with open fractures, those with fracture fragments incarcerated in the joint, concomitant elbow dislocation, those in whom a concentric reduction of the elbow joint cannot be achieved, and possibly valgus stress athletes with displaced fractures (Table 2).
Table 2
Indications/contraindications for non-operative management of medial epicondyle fracture
Indications | Contraindications |
|---|---|
Minimal displacement | Open fracture |
Fracture fragment incarcerated in joint | |
Inability to achieve concentric joint reduction |
Technique
In those patients to be managed without surgery, immobilization in a long arm cast with the elbow flexed 90° and neutral forearm rotation for 4 weeks is generally sufficient, though radiographic union is not always the expected outcome, especially for displaced fractures (Fig. 3). Once the period of immobilization is complete, range of motion exercises can be initiated with a gradual return to full activity once range of motion is maximized. For those patients with the fracture fragment incarcerated in the joint, a nonoperative reduction technique has been described. The Roberts maneuver involves a valgus force to open the joint, followed by forearm supination and finger extension (Roberts 1934). However, most now consider an incarcerated fracture fragment to be an indication for operative treatment, so the usefulness of the Roberts maneuver may be questioned.
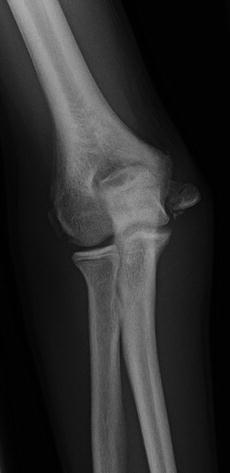

Fig. 3
AP radiograph showing a healed medial epicondyle fracture with at least partial bony healing
Outcomes
Traditionally, nonsurgical management of humeral medial epicondyle fractures largely yields good to excellent results (Josefsson and Danielsson 1986; Farsetti et al. 2001). A 1975 study demonstrated that almost 80 % of nonoperatively treated fractures had a functional, pain-free, stable, non-deformed elbow with less than a 15° loss of range of motion (Bede et al. 1975). Similarly, a 2007 study demonstrated that no patient treated nonoperatively had loss of strength or range of motion and had similar outcomes scores to those treated surgically (Ip and Tsang 2007). A systematic review found a trend towards less pain in those patients treated nonoperatively (Kamath et al. 2009a).
Operative Treatment
Indications and Contraindications
There are very few absolute indications for operative treatment of medial epicondyle fractures (Table 3). The only absolute indications are open fractures and fractures incarcerated in the joint (Aitken and Childress 1938; Fowles et al. 1984b, 1990; Dias et al. 1987; Wilson et al. 1988; Farsetti et al. 2001; Gottschalk et al. 2012). Relative indications for operative intervention are much broader. Measured displacement on radiographs is inconsistent (Edmonds 2010; Pappas et al. 2010; Gottschalk et al. 2013), and no cutoffs for acceptable displacement have been established (Smith 1950; Josefsson and Danielsson 1986; Hines et al. 1987; Fowles et al. 1990; Lee et al. 2005; Smith et al. 2010).
Table 3
Indications/contraindications for operative management of medial epicondyle fracture
Absolute indications | Relative indications | Contraindications |
|---|---|---|
Open fracture | Ulnar nerve dysfunction | Minimal displacement |
Fragment incarcerated in joint | Valgus instability | |
Inability to achieve concentric joint reduction | High-demand patient |
Ulnar nerve dysfunction is often considered an indication for operative fixation (Roberts 1934; Patrick 1946; Josefsson and Danielsson 1986; Farsetti et al. 2001). Significant ulnar nerve symptoms in the acute setting raise concerns that the ulnar nerve is impinging in the fracture site or within the elbow joint. Moreover, late ulnar nerve symptoms secondary to compromise of the ulnar nerve’s normal path around the medial epicondyle occur in approximately 5 % of nonoperatively treated fractures (Josefsson and Danielsson 1986; Farsetti et al. 2001) and can occur up to 3–9 months after the injury (Royle and Burke 1990). However, a systematic review found that there were no differences in outcomes for patients with preoperative ulnar nerve symptoms that were treated surgically versus nonsurgically (Kamath et al. 2009a).
Valgus instability has frequently been cited as an indication for operative treatment of medial epicondyle fractures given the likely associated soft tissue injuries and potential implications on long-term outcomes (Smith 1950; Kilfoyle 1965; Louahem et al. 2010). Some authors argue that all patients with a medial epicondyle fracture should undergo valgus stress testing to look for medial instability (Schwab et al. 1980; Gottschalk et al. 2012).
The third relative indication is a medial epicondyle fracture in high-demand valgus stress athletes such as pitchers and gymnasts (Kamath et al. 2009a). However, it has been pointed out that at the age of 11 or 12 it is often difficult to determine which patients will be high-level athletes (Mehlman and Howard 2012). A 2013 case series of 20 athletes compared patients who underwent operative and nonoperative treatment of their medial epicondyle fractures (Lawrence et al. 2013). Patients were treated nonoperatively if their injury resulted from a low-energy injury and the patient had no evidence of elbow instability on valgus stress testing. The authors did not employ a strict cutoff for the amount of acceptable displacement. Of the 14 patients treated nonoperatively, all were able to return to their sports at the next appropriate level and none of the patients felt that their performance was limited after treatment.
Techniques
Numerous techniques for the operative treatment of medial epicondyle fractures have been described including suture repair (Wilson et al. 1988; Fowles et al. 1990; Lee et al. 2005), Kirschner (K-) wire fixation (Papavasiliou 1982; Hines et al. 1987; Wilson et al. 1988; Fowles et al. 1990; Farsetti et al. 2001; Lee et al. 2005; Ip and Tsang 2007; Louahem et al. 2010; Park and Kwak 2012), screw fixation (Wilson et al. 1988; Fowles et al. 1990; Case and Hennrikus 1997; Lee et al. 2005; Haxhija et al. 2006; Kamath et al. 2009a, b; Park and Kwak 2012), and excision of the epicondylar fragment and suturing the soft tissue to the periosteum of the medial elbow (Farsetti et al. 2001; Gilchrist and McKee 2002). Screw fixation is the most often utilized technique today. This treatment allows for an anatomic reduction, rigid fixation, and early mobilization (Gottschalk et al. 2012). However, K-wires have also been utilized with similarly good results (Park and Kwak 2012). Wire migration, although a rare phenomenon, must be monitored (Cheng et al. 1997; Sykora et al. 2013). Excision of the epicondylar fragment has been known to lead to persistent elbow pain despite suturing the soft tissue back to the periosteum of the medial elbow (Farsetti et al. 2001) and has largely fallen out of favor.
Surgical Procedure
Preoperative Planning
See Table 4.
Table 4
Preoperative planning for screw fixation of medial epicondyle fracture
OR table | Hand table attachment |
Position | Supine |
Lateral | |
Prone (Glotzbecker et al. 2012) | |
Fluoroscopy | From patient’s head |
Equipment | 4–0 cannulated screw set +/− washers |
Tourniquet | Sterile tourniquet |
Misc | Esmarch to aid in reduction (Kamath et al. 2009b) |
Positioning
Patients can be positioned in several different ways to maximize access to the medial aspect of the elbow. A supine position can be used with the patient’s entire operative extremity on a hand table (Kamath et al. 2009b). Alternatively, the patient may be positioned laterally with the operative arm draped across the patient and the surgical team standing towards the patient’s foot. Finally, a prone position may be employed. A hand table is used and the arm is kept internally rotated, flexed, and pronated (Glotzbecker et al. 2012).
Fluoroscopy setup is based on surgeon preference and patient positioning. While bringing fluoroscopy in from the patient’s head is common, other methods can be employed, with the focus on obtaining the maximum amount of space available to work. Given the superficial location of the epicondyle, a tourniquet can be considered optional, but the authors find it useful. If a tourniquet is used, it may be either sterile or non-sterile. In smaller children, a sterile tourniquet may be preferable, as it tends to increase the available surgical field relative to a non-sterile tourniquet after prepping and draping.
Surgical Approach
Medial epicondyle fractures are accessed via a posteromedial approach that is centered just anterior to the anatomic position of the medial epicondyle (Kamath et al. 2009b), though an incision just posterior to the medial epicondyle allows better exposure of the nerve and may be more cosmetic. The incision should allow for exposure of the fracture site, the fracture fragment, and the ulnar nerve (Fig. 4). The medial epicondyle fragment is usually displaced anteriorly and distally. Careful debridement of the fracture bed should be undertaken and obstructions to fracture reduction should be removed to allow for an anatomic reduction. The ulnar nerve should be identified and kept out of harm’s way with a vessel loop (Fig. 4). If there are preoperative ulnar nerve symptoms, identification and dissection of the ulnar nerve is highly recommended.
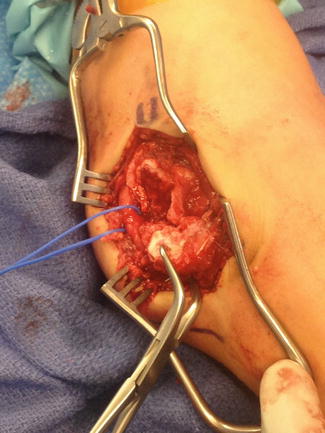

Fig. 4
Intraoperative photo of operative fixation of a medial epicondyle fracture. The exposure is wide in this case to fully expose and protect the ulnar nerve. In this case, the patient had a dense preoperative ulnar nerve palsy. The fracture fragment is grasped with a pointed reduction clamp and the fracture bed is fully exposed for later reduction
Surgical Technique
To reduce the fracture fragment back to the fracture bed, wrist flexion relaxes the flexor wad attached to the fracture fragment (Table 5). Additionally, an Esmarch bandage can be applied from the distal to proximal direction to allow for gentle “milking” of the soft tissues and the fragment towards the humerus (Kamath et al. 2009b). While making certain the ulnar nerve is out of harm’s way, provisional reduction is often achieved with some variety of a pointed instrument such as a dental pick, 18-gauge needle (Kamath et al. 2009b), pointed reduction forceps, or towel clamp (Fig. 4). The guidewire for the cannulated screw can be used as well to “joystick” the fracture into position. A narrow gauge K-wire can be used to transfix the fragment to the humerus in order to prevent rotation as the guidewire for the cannulated screw is advanced (Kamath et al. 2009b; Fig. 5). Great care must be taken with this technique, particularly with small fragments of bone that may be easily split.
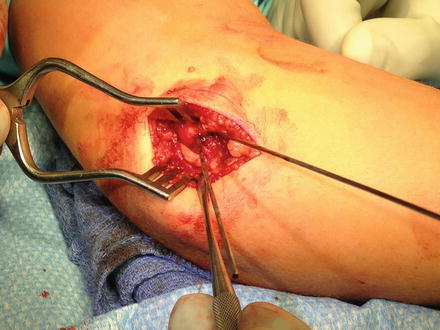
Table 5
Surgical steps
Posteromedial incision |
Identify and protect ulnar nerve |
Identify displaced fracture fragment |
Reduce fragment |
Transfix fragment to distal humerus |
Pass guidewire in superolateral direction |
Place 4.0 mm cannulated partially threaded screw (+/− washer) |
Reattach soft tissues to medial epicondyle |
Irrigate and close |

Fig. 5
Intraoperative photo depicting provisional reduction and fixation with a K-wire while the cannulated guidewire is also in place. The ulnar nerve is also exposed at the posterior aspect of the incision, but it is not widely dissected due to an intact preoperative neurological exam
The guidewire for the cannulated screw should be advanced in a superolateral direction between the medial cortex and the olecranon fossa (Fig. 6a and b). Since the medial epicondyle is both posterior and medial, the guidewire should be directed anterior enough to stay within the distal shaft of the humerus. The screw should be directed up the lateral column. Avoid the pitfall of a screw in the olecranon fossa, which acts as a mechanical block to extension (Fig. 7a–b). In addition, the guidewire should not penetrate the far cortex, as the radial nerve is at risk of iatrogenic injury (Marcu et al. 2011). Once the guidewire position is confirmed on AP and lateral fluoroscopic images, the near cortex is breached with a 2.7 mm drill, and a 4.0 mm partially or fully threaded cancellous screw is placed. Care should be taken not to apply too much compression, or risk splitting the fracture fragment. In very hard bone one may want to consider drilling the proximal screw path as well. A washer can be used to increase the surface area for compression and prevent screw head penetration or fragmentation of the piece. However, inclusion of a washer increases screw head prominence and may result in an increased need for hardware removal.
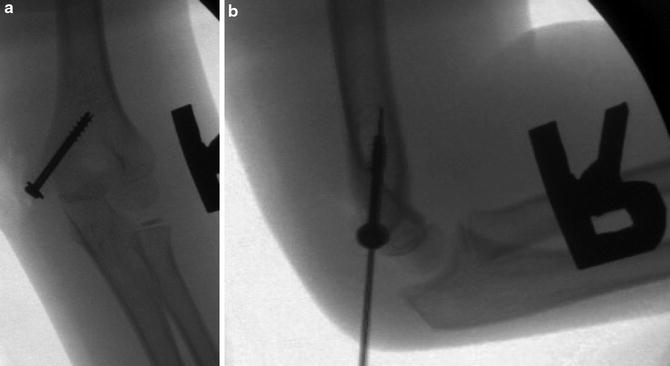
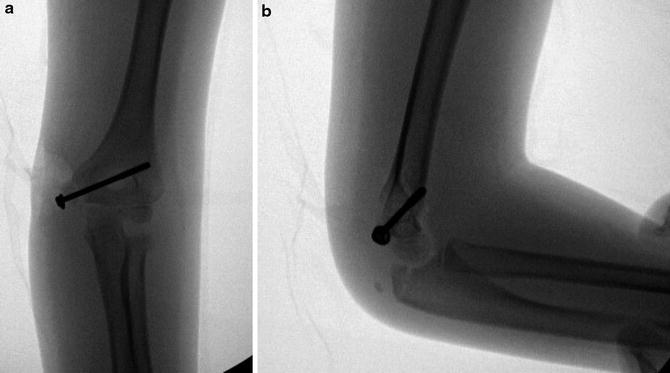

Fig. 6
Appropriate screw placement on (a) AP and (b) lateral radiographs

Fig. 7
Inappropriate screw placement on (a) AP and (b) lateral radiographs. The screw is too horizontal and crosses into the olecranon fossa, which may cause a mechanical block to extension
Postoperative Protocol
Postoperatively, patients are immobilized in a long arm cast versus a posterior splint for 1–2 weeks to allow the soft tissues to heal (Table 6). A simple sling is also an option, but may increase pain after surgery. After that time, immobilization is removed and patients are encouraged to begin range of motion exercises. The use of a hinged elbow brace can be utilized to protect any healing medial soft tissue structures. Strengthening exercises can typically begin 4–6 weeks after surgery, and most patients can return to sports, including throwing, 3 months after surgery.
Table 6
Postoperative protocol
Type of immobilization | Long arm cast |
Length of immobilization | 2 weeks |
Rehab protocol | Gentle active range of motion, advance to active assist as pain allows |
Return to sports | Once full motion returns |
Outcomes
In general, quality outcomes data are sparse. Early outcome studies show good functional results in 62.5 % of surgically treated fractures (Bede et al. 1975). When patients were compared using the Mayo Clinic Performance Index, an instrument which emphasizes pain and activities of daily living, both groups achieved high average scores, and there was no significant difference between the two groups (Ip and Tsang 2007). Most patients are able to return to their previous level of activity after treatment for a medial epicondyle fracture. In a case series of 20 athletes treated surgically for medial epicondyle fractures and followed for greater than 2 years, all of the patients achieved excellent outcomes and all of the overhead athletes were able to return to their sport at the next appropriate level (Lawrence et al. 2013). None of the patients felt that their performance was limited after treatment. However, in a large meta-analysis, operatively treated patients also trended towards more pain at final follow-up when compared with those patients treated nonoperatively (Kamath et al. 2009a). Given the nature of the study, the authors were unable to comment on the reason(s) for the increased pain (prominent hardware, higher-energy injury with more soft tissue damage, etc.).
Preferred Treatment
In determining whether a medial epicondyle fracture should be treated operatively or nonoperatively, several factors need to be taken into account. First is the presence of a concomitant elbow dislocation. Patients with a medial epicondyle fracture and an elbow dislocation have an inherently more unstable fracture pattern with more soft tissue damage and are thus more likely to benefit from operative intervention. The second factor is the amount of displacement seen. Minimally displaced fractures (less than 5–10 mm) can be treated conservatively in the absence of an associated elbow dislocation. Generally, patients with greater than 10 mm of displacement, an associated elbow dislocation, and ulnar nerve symptoms or high-demand valgus stress athletes are considered appropriate surgical candidates.
Surgical intervention is generally carried out as described above with the patient in the supine position and the operative extremity on a hand table. The fluoroscopy machine is brought in from the head to allow the surgical team to sit in the region of the patient’s axilla. Dissection, reduction, and fixation are carried out as described above. Use of a washer is based on surgeon preference. Use of a washer increases screw head prominence and decreases patient comfort; however, its use may minimize the chance of fracture fragmentation. Patients are immobilized for a period of generally 1 week to allow for soft tissue healing before beginning range of motion exercises. Strengthening is initiated at 3–4 weeks postoperatively. Patients are allowed to return to sports typically 3 months postoperatively. Screw removal is based on surgeon preference. Some prefer to leave hardware in unless symptomatic, while others prefer to routinely remove hardware at 3–6 months postoperatively if the patient has significant growth remaining.
Complications
Complications in the treatment of medial epicondyle fractures include loss of motion, cubitus valgus, bony nonunion, and surgical complications such as infection and nerve and blood vessel damage. Loss of motion is commonly seen in both operatively and nonoperatively treated fractures. In nonsurgically treated patients loss of extension is reported in 20–30 % of patients with an average loss of extension of 10° or more. Less than 5 % will have an extension loss greater than 30° (Smith 1950; Josefsson and Danielsson 1986; Wilson et al. 1988). In those patients treated operatively, loss of extension was found to be higher than those treated without surgery (Fowles et al. 1990; Louahem et al. 2010). In 1990, Fowles noted an average loss of 37 degrees of extension loss in patients who underwent operative treatment for a medial epicondyle fracture with an associated elbow dislocation compared with only 15° in the nonsurgical group (Fowles et al. 1990). Similarly, Hines et al. (1987) noted a loss of range of motion in patients requiring arthrotomy to remove an interposed fracture fragment, which was unrelated to the degree of displacement, the time from injury to surgery, or the duration of immobilization. However, this difference may be secondary to the fact that those patients who underwent surgery sustained a more severe injury with increased risk of elbow stiffness from the injury itself. Additionally, most of the cited studies are somewhat dated and operative techniques and rehabilitation protocols have changed considerably. The authors have not found significant loss of motion if therapy is started early.
Recent small case series have reported on patients with symptomatic valgus instability after nonoperative treatment (Gilchrist and McKee 2002; Smith et al. 2010). Gilchrist and McKee (2002) published a report on three pediatric patients with symptomatic valgus instability secondary to long-standing medial epicondyle nonunions. A second case series reported on eight patients with medial epicondyle nonunions, all of whom experienced ongoing pain (Smith et al. 2010). Half of these patients had concomitant valgus instability of the elbow, and all of these patients were successfully treated with open reduction and screw fixation with selective use of bone grafting. The postoperative rate of valgus instability is less than 1 % (Lee et al. 2005; Ip and Tsang 2007; Louahem et al. 2010; Mehlman and Howard 2012).
Nonunion in patients treated nonoperatively is of unclear significance. In a recent meta-analysis, patients who were treated nonoperatively had only a 49.2 % rate of bony union (Kamath et al. 2009a). Other studies have identified a nonunion rate of more than 60 % when medial epicondyle fractures are treated nonoperatively, all with excellent clinical results and no ongoing issues of instability (Josefsson and Danielsson 1986; Wilson et al. 1988; Farsetti et al. 2001). In comparison, a systematic review found a 92.5 % rate of bony union in patients treated surgically (Kamath et al. 2009a), and a pooled analysis of recent studies found only one out of 188 patients treated surgically for a medial epicondyle fracture failed to achieve a bony union (Lee et al. 2005; Ip and Tsang 2007; Louahem et al. 2010; Mehlman and Howard 2012).
Cubitus valgus has been reported in up to 35.5 % of patients (Bede et al. 1975) and is a phenomenon of nonoperatively treated fractures. Most cases are mild and not clinically significant, with less than 10 % of patients demonstrating substantial valgus deformity (Pimpalnerkar et al. 1998; Farsetti et al. 2001; Lee et al. 2005). Due to the relatively sparse literature, it is unknown if this is a clinically significant problem.
Surgical complications are rare in operatively treated medial epicondyle fractures (Bede et al. 1975; Hines et al. 1987; Wilson et al. 1988; Ip and Tsang 2007; Marcu et al. 2011; Mehlman and Howard 2012). The infection rate seen with these fractures is approximately 0.5 % (Lee et al. 2005; Ip and Tsang 2007; Louahem et al. 2010; Mehlman and Howard 2012). There have been no reports to date of late ulnar nerve symptoms seen postoperatively (Mehlman and Howard 2012); however, a case series exists describing two iatrogenic radial nerve injuries secondary to far cortex penetration by a guidewire or screw (Marcu et al. 2011).
Transphyseal Fractures
Introduction
Transphyseal distal humerus fractures are a rare fracture pattern. The first complete description of this injury that differentiated it from supracondylar humerus fractures and elbow dislocations appeared in 1850 (Smith 1850). However, prior to the 1970s, these fractures were largely described in individual case reports (Marmor and Betchol 1960; Siffert 1963; Mauer et al. 1967; Omer and Simmons 1968; Kaplan and Reckling 1971). Later series eventually reported on a total of 45 transphyseal distal humerus fractures (Mizuno et al. 1979; DeLee et al. 1980; Holda et al. 1980; Peiro et al. 1981; McIntyre et al. 1984; Akbarnia et al. 1986; de Jager and Hoffman 1991). Even with that, data on this fracture is sparse.
Transphyseal fractures tend to occur in neonates and young children, with almost all reports in patients younger than 4 years of age (DeLee et al. 1980; Moucha and Mason 2003). The peak incidence is seen from newborn to two and a half years old, and there are no reports in patients older than 7 years (DeLee et al. 1980).
The true incidence of these fractures is likely underreported, as they tend to occur in very young patients and often go unrecognized. One series found that 50 % of fractures that were eventually diagnosed as a transphyseal fracture were not correctly diagnosed until 1–6 days after the injury (DeLee et al. 1980). Prompt diagnosis and treatment of these fractures requires a high index of suspicion.
Pathoanatomy and Applied Anatomy
At birth, the entire distal humerus is cartilaginous. Secondary ossification centers in the distal humerus do not appear until approximately 1–11 months of age in girls and 1–26 months in boys (Haraldsson 1959). The distal humeral physes fuse to one another at 10–12 years of age and fuse to the distal humerus by 12–16 years of age (Haraldsson 1959).
The volume of the distal humerus occupied by the distal epiphysis is larger in younger children. As the humerus matures, the physeal line progresses more distally with a central “V” forming between the medial and lateral condylar physes. This “V”-shaped configuration of the physeal line helps to protect the more mature distal humerus from physeal fractures (Ashurst 1910).
The blood supply to the medial crista of the trochlea courses directly through the physis (Haraldsson 1959). As such, the blood supply to this area is vulnerable to injury with transphyseal fractures. Injury to these vessels places the patient at risk for osteonecrosis in the medial crista of the trochlea.
Mechanism of Injury
The exact mechanism of injury for these fractures is unknown and likely varies by age. In neonates, a transphyseal fracture can present as a birth injury (Siffert 1963; Berman and Weiner 1980; Downs and Wirth 1982; Barrett et al. 1984; Akbarnia et al. 1986). Some authors postulate that because the physeal line is more proximal in young infants and nearer to the center of the olecranon fossa, a hyperextension injury in this age group is more likely to result in a physeal separation as opposed to a bony supracondylar fracture (Dameron 1981). In older children, with a relatively more distal physis, a hyperextension injury to the elbow concentrates the forces generated by the olecranon in the supracondylar area of the humerus, resulting in a supracondylar humerus fracture (DeLee et al. 1980).
Others argue that infants and younger children tend to have some residual flexion contracture of the elbow from intrauterine positioning, and these contractures may prevent the hyperextension-type mechanisms (DeLee et al. 1980). Previous studies have shown that the physis is more likely to fail with rotatory shear forces than with pure bending or tension forces (Bright et al. 1974). In younger children, forces applied to the elbow are more likely to be rotatory shear, such as those secondary to birth trauma or child abuse (DeLee et al. 1980).
Most importantly, many reports have identified a very high incidence of confirmed or suspected child abuse in infants and young children with these injuries (DeLee et al. 1980; Akbarnia et al. 1986; Willems et al. 1987; de Jager and Hoffman 1991). One series found that out of 16 transphyseal fractures, six were secondary to confirmed or suspected child abuse (DeLee et al. 1980).
Assessment
Clinical Presentation
A careful history is required in all patients suspected of having this fracture. Birth trauma is a common cause of transphyseal fractures. However, in all patients in whom this is not a viable mechanism, child abuse must be ruled out (DeLee et al. 1980; Akbarnia et al. 1986; Willems et al. 1987; de Jager and Hoffman 1991). Previous reports have confirmed or suspected child abuse as the mechanism for transphyseal fractures in up to a third of patients being evaluated (DeLee et al. 1980; Akbarnia et al. 1986; Willems et al. 1987; de Jager and Hoffman 1991).
The clinical presentation of transphyseal fractures can be variable by age. Birth injuries at the time of delivery resulting in these fractures tend not to have especially impressive exams, with only moderate swelling and little crepitus being reported (Siffert 1963). Often, the only sign present in newborns with this injury may be pseudoparalysis of the involved limb. Careful clinical examination and radiographic evaluation are required to differentiate this injury from a brachial plexopathy and/or other birth fractures. As young children may have only minimal swelling and this fracture is easily missed, transphyseal fractures should be suspected in any infant under 18 months of age whose elbow is swollen secondary to trauma (Siffert 1963).
In younger children, there may be marked swelling apparent, with an elbow that appears “dislocated” on radiographs (DeLee et al. 1980). If palpable, the relationship, however, between the epicondyles and the olecranon is maintained (Fig. 8a). Rotatory deformity, as opposed to angular deformity, is typically seen with these fractures as the fracture fragments have a wide surface area, which minimizes tilting of the distal fragment (Fig. 8b). Poland described a “soft crepitus” present with transphyseal fractures (DeLee et al. 1980). Gentle manipulation of the forearm against the humerus produces abnormal motions about the elbow joint and a muffled crepitus. This occurs as motion between the two cartilaginous fracture fragments produces a crepitus distinct from that produced with two bony fragments (DeLee et al. 1980). This muffled crepitus is considered diagnostic of a physeal separation.
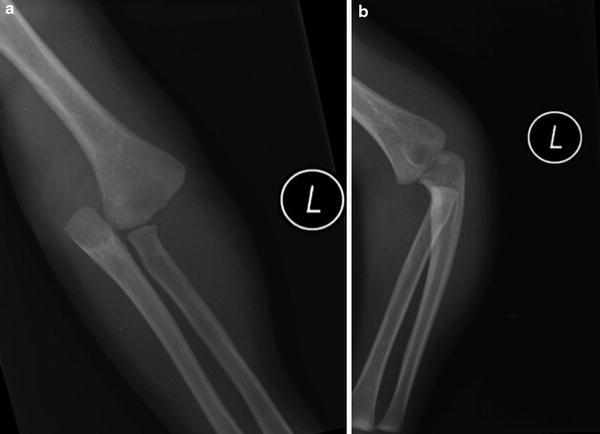

Fig. 8
(a) AP of a transphyseal fracture. No ossification of the humeral epiphysis is present and this could easily be mistaken for an elbow dislocation. (b) A lateral radiograph shows a lateral image of the proximal forearm but a near AP of the distal humerus. This nicely depicts the rotatory displacement that is often characteristic of transphyseal fractures and is one of the distinguishing characteristics from an elbow dislocation
Radiographic Evaluation
Radiographic evaluation of these fractures can be difficult, especially if the ossification center of the capitellum is not visible yet, as in an infant. Although the distal humerus cannot be seen on routine radiographs in extremely young patients, its location can be inferred from the position of the ossified portions of the proximal radius and ulna. However, in patients who do not yet have a capitellar ossification center, other imaging may be needed to confirm the diagnosis of this fracture, including magnetic resonance imaging (MRI), ultrasound (US), or an elbow arthrogram (Mizuno et al. 1979; Hansen et al. 1982; Barrett et al. 1984; Akbarnia et al. 1986).
These fractures can be difficult to distinguish from other pediatric elbow injuries (DeLee et al. 1980), namely, elbow dislocations. In addition, transphyseal fractures can also be confused with other injuries including supracondylar fractures, lateral condyle fractures, or Monteggia fractures. All reports of transphyseal fractures, with rare exceptions (Berman and Weiner 1980), demonstrate posteromedial displacement of the distal fragment (Marmor and Betchol 1960; Siffert 1963; Mauer et al. 1967; Omer and Simmons 1968; Kaplan and Reckling 1971; Sutherland and Wrobel 1974; DeLee et al. 1980; Oh et al. 2000). Elbow dislocations tend to be lateral, with a medial dislocation being extremely rare (Linscheid and Wheeler 1965; Roberts 1969; Neviaser and Wickstrom 1977; Royle 1991). Additionally, as most transphyseal fractures in older children have a small flake of metaphyseal bone, or “corner sign,” with the distal fragment (Fig. 9), the absence of a bony fragment in older children can assist in distinguishing an elbow dislocation from a transphyseal fracture (DeLee et al. 1980). On the radiograph of a normal elbow, a line drawn down the long axis of the radius passes through the capitellum (Smith 1947, 1967; Storen 1959; Rogers 1978; Ruo 1987). This relationship should be maintained in all elbow fractures and is disrupted only in dislocations that include disruption of the radiohumeral joint. Confirmation of the injury can be performed with live fluoroscopy under sedation. As the forearm is manipulated anteriorly and posteriorly, the medial and lateral condyles of the humerus (if ossified) and olecranon maintain a constant relationship to each other (DeLee et al. 1980). This clearly differentiates a physeal separation from an elbow dislocation. Finally, transphyseal fractures tend to occur in neonates and young children (DeLee et al. 1980; Holda et al. 1980; Akbarnia et al. 1986; de Jager and Hoffman 1991), while elbow dislocations are much more common in children nearing skeletal maturity (Henrikson 1966; Josefsson and Nilsson 1986).
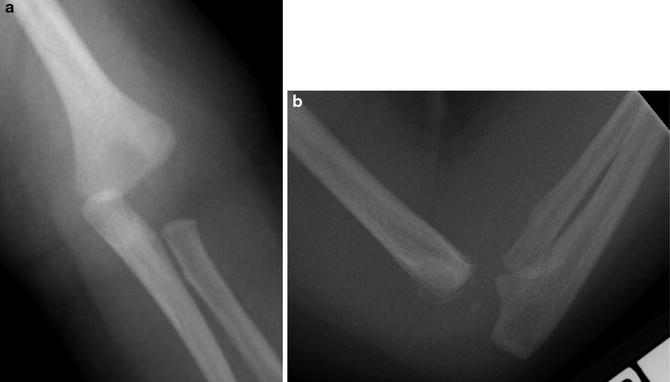

Fig. 9
(a) AP radiograph of a transphyseal fracture in the setting of child abuse. The capitellar ossification center is just now forming. The forearm has an abnormal relationship with the distal humeral metaphysis but the proximal radius and the capitellum are in line with each other. (b) The lateral radiograph helps confirm the diagnosis with the small metaphyseal flake of bone characteristic of a transphyseal fracture
The key distinguishing features of a supracondylar fracture are that the entire fracture line is visible in the supracondylar area above the epiphyseal plate and the relationship between the proximal forearm and humeral epiphysis is maintained. Some transphyseal fractures are actually SH II injuries with a small flake of metaphyseal bone. This can be confused with a lateral condyle fracture. However, in lateral condyle fractures, the radius will maintain its relationship with the intact portion of the capitellum, while the fractured portion displaces laterally. In transphyseal fractures, the flake of bone will stay with the proximal radius (Fig. 9a and b). With Monteggia fractures, the relationship between the radius and ulna is altered, while the relationship between the ulna and humeral epiphysis, and therefore with the distal humeral metaphysis, is maintained.
Classification
No single, validated, widely used classification system exists for transphyseal fractures of the distal humerus (Table 7). DeLee and colleagues proposed a classification system based on the degree of ossification of the lateral condyle epiphysis (DeLee et al. 1980). Group A is infants up to 12 months of age, prior to the appearance of the lateral condyle epiphysis. No metaphyseal fragment is seen on the distal fragment. These fractures are usually Salter-Harris type I fractures and are often missed secondary to the lack of ossification in the lateral epiphysis. Group B is children 12 months to 3 years of age with definitive ossification of the lateral condyle epiphysis. These patients may have a small flake of metaphyseal bone on the distal fragment (Fig. 9a and b). Group C is older children 3–7 years of age. These patients have a large Thurston-Holland-type metaphyseal fragment. These fragments are most commonly lateral, though they may be medial or posterior.
Age | Lateral condylar epiphysis ossification | Metaphyseal fragment | |
|---|---|---|---|
Group A | <12 months | No | No |
Group B | 12 months to 3 years | Yes | Minimal |
Group C | 3 years to 7 years | Yes | Large |
Management
Nonoperative Management
Indications/Contraindications
There are very few indications for nonoperative management of transphyseal distal humerus fractures (Table 8). The first is delayed presentation or recognition. If the patient does not present until 5–6 days after the initial injury, the epiphysis is usually no longer mobile, and callus is already present (DeLee et al. 1980). Attempted manipulation at this point puts the physis at increased risk for further injury and will likely not result in an improved outcome. In addition, neonates or very small infants in whom general anesthesia or pin fixation may be difficult may be suitable candidates for nonoperative management.
Table 8
Indications/contraindications for operative management of transphyseal distal humerus fractures
Absolute indications | Contraindications |
|---|---|
Open fracture | Delayed presentation |
Significant fracture displacement | Neonates |
Techniques
For patients who present in a delayed fashion, no attempts should be made at reduction after 5–7 days, depending on the age of the patient. These patients should be splinted or casted for comfort for a total of 3 weeks from the time of injury. It is better to allow these patients to heal and treat any resulting deformity with a supracondylar osteotomy than to risk physeal injury or osteonecrosis of the physis (DeLee et al. 1980; Holda et al. 1980; Morrissy and Wilkins 1984; Moucha and Mason 2003). In neonates who are felt to be too young to undergo closed reduction and pinning, the affected extremity may be immobilized in 90 degrees of flexion with the forearm pronated for posterior medial displacement – while being very cognizant of swelling and the risk of compartment syndrome. The extremity may be stabilized with a figure-of-eight splint or a cast if size permits. Total duration of immobilization should be approximately 3 weeks.
Outcomes
In patients younger than 2 years old, restoration of appearance and function can be complete even without treatment (DeLee et al. 1980). Many essentially untreated transphyseal separations remodel completely without any residual deformity if the distal fragment is only medially translated and not tilted. Most of these fractures have very little tilt as the fracture fragments have wide surfaces on which to rest, minimizing the varus or valgus angulation. Moreover, because the intra-articular surface is not involved, complete functional recovery can usually be expected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


