and Sunil K. Sinha14
(13)
Division of Neonatal-Perinatal Medicine, F4790 C.S. Mott Children’s Hospital, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5254, USA
(14)
Paediatrics and Neonatal Medicine, The James Cook University of Hospital, University of Durham, Middlesbrough, UK
8.1.1.1 Introduction
A mechanical ventilator is an automated device that provides all or part of the work of breathing for patients with impaired respiratory or neurologic function. In order to safely apply a mechanical ventilator to a patient for continuous use, four requisites must be met (Table 8.1). First, there must be a way to create a stable attachment of the device to the patient, referred to as the interface. Second, there must be an energy source to drive the device. Third, the size and timing of the inflation must be regulated or controlled. Fourth, there must be a system to adequately monitor the performance of the ventilator and the status of the patient. This should include adjustable alarms to alert the clinician to undesirable and potentially dangerous conditions Chatburn (2003).
Table 8.1
Requisites for mechanical ventilators
1. Stable patient–ventilator interface |
2. Energy source |
3. Control and regulation of size and timing of inflation |
4. Monitoring and alarm system |
8.1.1.2 Mechanical Ventilators
Mechanical ventilators can be broadly classified into two major categories based upon the size of the delivered inflation (Table 8.2). Conventional mechanical ventilators deliver tidal volumes, which are within the normal physiological range. This is referred to as tidal ventilation, and it comprises the majority of mechanical ventilatory devices. High-frequency ventilators deliver much smaller tidal volumes. (Bunnell 2006). Actual measurements show the VT during HFOV is often > 2 ml/kg in patients with significant lung disease (Zimová-Herknerová and Plavka 2006). These will be considered in more detail in a subsequent chapter.
Table 8.2
Classification of mechanical ventilators
Conventional mechanical ventilator (tidal) |
Negative-pressure ventilation |
Positive-pressure ventilation |
Continuous flow |
Pressure limited |
Variable flow |
Pressure control |
Pressure support |
Constant flow |
Volume targeted |
Hybrids |
High–frequency ventilation (nontidal) |
Jet ventilation |
Oscillatory ventilation |
Flow interruptors or percussive ventilators such as the Bronchotron |
Conventional mechanical ventilators may also be subdivided based upon the method by which gas flow is introduced into the airway and lung. A gradient may be established by the application of positive pressure, in which gas is delivered to the patient through a circuit. Conversely, the gradient can be achieved through the creation of negative pressure. The patient is placed inside an incubator or chamber and sealed from the neck down. Pressure within the chamber is cyclically decreased (usually by a vacuum pump), forcing gas to enter the airway. Negative-pressure devices, such as the iron lung, were commonly used during the poliomyelitis epidemic of the 1950s but are seldom utilized today. Thus, the overwhelming majority of mechanical ventilation performed on pediatric populations is provided by conventional positive-pressure ventilators.
Positive-pressure ventilation is usually powered by an electrical or compressed gas source. Electricity may be used to run compressors, which in turn create the driving force for ventilation. Compressed gas may also come from separate sources, such as tanks or wall outlets, allowing the blending of air and oxygen (Chatburn 2003). Because compressed gas is devoid of humidity and is damaging to the respiratory epithelial tissues, a heated source of humidification is added to the ventilator circuit (Schulze 2006).
The control system is used to be sure that the patient receives the desired pattern of respiration. The clinician chooses the pressure (peak inflation pressure, PIP) or volume (tidal volume, V T) to be delivered, the baseline pressure (positive end-expiratory pressure, PEEP), the mandatory rate of mechanical inflation, how long the breath lasts (inspiratory time, T i), and how much effort the patient has to exert (assist sensitivity) to trigger the ventilator. If the breath is triggered by the patient, it is referred to as a spontaneous breath; if it is initiated by the ventilator, it is referred to as a mandatory or control breath. Ventilator modes refer to the pattern of how spontaneous and mandatory inflation are delivered to the patient (Chatburn 2003).
Monitoring systems consist of both alarms, which notify clinicians when set parameters have been breached, and data displays, which may be digital or graphic. Alarms may signal disconnection of the patient from the ventilator or the ventilator from its power source. Circuit control alarms may indicate electronic failures or ventilator settings which may be incompatible with ventilator function. Output alarms may warn of pressure, volume, or flow that exceeds or cannot meet the preset limits. Graphic displays include waveforms (or scalars) for flow, volume, and pressure over time, and numerous pulmonary “loops,” such as pressure volume or flow volume. Digital displays exist for multiple parameters, including peak pressure, end-expiratory (or baseline) pressure, inspiratory time, rate, and tidal volume. Many devices are also capable of calculating physiological measurements, such as mean airway pressure and minute ventilation, or pulmonary mechanics measurements, such as dynamic compliance or resistance. Storage of data and displays of trends over time may assist in the interpretation of data and management of the patient (Fig. 8.1).
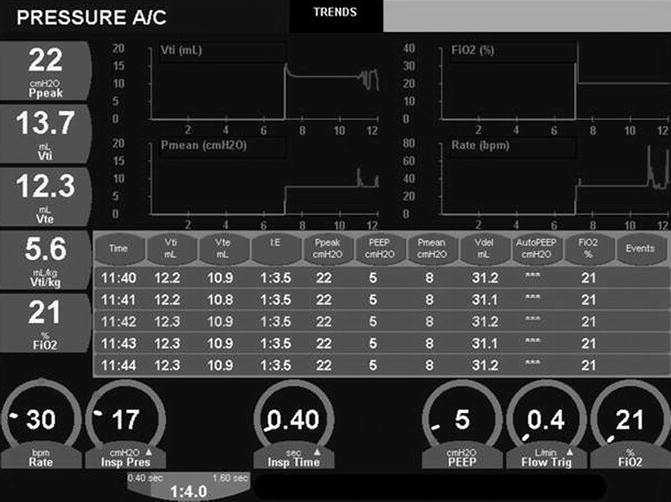

Fig. 8.1
Trend monitoring. Both graphic and analog data are trended here on a minute-to-minute basis
8.1.1.3 Continuous-Flow Systems
The creation of a device that offered continuous flow in the ventilator circuit enabled the development of neonatal mechanical ventilation. Because the intrinsic respiratory rate of the newborn is high relative to an older child or adult, the baby required a source of fresh gas to breathe between mechanical inflation. This is referred to as bias flow, and the rate is set by the clinician. When the ventilator exhalation valve closes during inspiration, the bias flow is diverted to the patient and the lungs are actively inflated. At the end of inspiration, the valve opens, and the lungs are passively deflated by elastic recoil (Fig. 8.2).
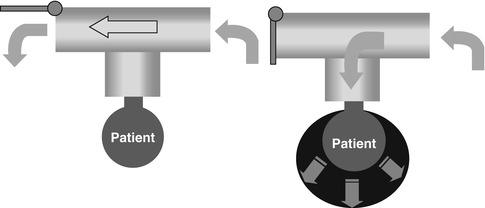

Fig. 8.2
Schematic drawing of continuous-flow, intermittent mandatory ventilation. On the right side, expiratory valve closes and gas flow is diverted to the baby, filling the lungs. On the left side, inspiration has ended, the exhalation valve has opened, and the lungs are passively deflating
The flow rate (in L/min) should be set high enough to allow the ventilator to reach the PIP in the allotted time. If it is set too low, the patient may develop air hunger and increased work of breathing. If it is set too high, it can create turbulence and ineffective gas exchange, lead to inadvertent PEEP, and result in overdistension of the lungs. Inappropriate circuit flow may result in rheotrauma (Donn and Sinha 2006), a component of ventilator-induced lung injury (Attar and Donn 2002).
Continuous flow is used during pressure-limited ventilation. This was the most common method of providing mechanical ventilation to neonates for more than a quarter of a century. Since the advent of microprocessor-based technology, two new ways to provide gas flow have been introduced into neonatal respiratory care.
8.1.1.4 Variable-Flow Systems
Variable-flow ventilation can be accomplished by actually controlling inspiratory flow through the use of proportional solenoid valves. This creates an inspiratory flow waveform that has a sharply accelerating phase followed by a rapidly decelerating phase (Fig. 8.3). This flow pattern is utilized in pressure-controlled ventilation and pressure-support ventilation. It results in rapid pressurization of the ventilator circuit and rapid delivery of gas to the lung, with peak pressure and peak volume delivery occurring early in inspiration. It may be thought of as a “front-end loaded” breath. Intuitively, this should be beneficial in pathophysiological states characterized by homogeneous lung disease where compliance is low and resistance is high (Donn and Boon 2009). Because flow is variable, some devices offer a qualitative way to control it through an adjustable rise-time feature. This alters the slope of the inspiratory pressure waveform. If the rise time is too flat, air hunger may result. If it is too steep, pressure overshoot may occur. Careful adjustment helps to achieve the appropriate degree of hysteresis in the pressure–volume loop.
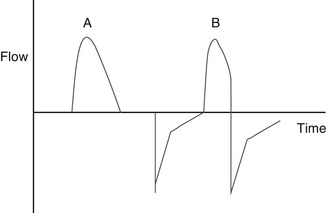

Fig. 8.3
Flow waveform, generated by measuring flow vs time. This is variable-flow ventilation, producing a rapidly accelerating then decelerating flow waveform. (A, B) On the left, breath is time cycled; on the left it is flow cycled
A major drawback of both continuous- and variable-flow ventilation is that although pressure is well controlled, volume will vary. At the same pressure, volume will be proportional to compliance. When the lung is stiff, tidal volumes will be low; when compliance improves, tidal volume will increase, and the clinician will need to make the appropriate adjustments.
8.1.1.5 Constant-Flow Systems
Constant-flow ventilation is utilized to provide volume-targeted or volume-controlled ventilation. Inspiratory flow accelerates at the start of inspiration but is held constant at the peak flow rate, creating a square flow waveform. This results in a ramping effect of both volume and pressure delivery, where both peak pressure and maximum volume delivery occur at the end of inspiration (Fig. 8.4). Thus, in contrast to pressure-targeted inflation, volume-targeted inflation are “back-end loaded.” They result in a slower inflation of the lung, and they may be more suitable to pathophysiological states characterized by nonhomogeneous lung disease, where rapid inflation would preferentially deliver more gas to the more compliant areas of the lung, creating or contributing to ventilation–perfusion mismatch.
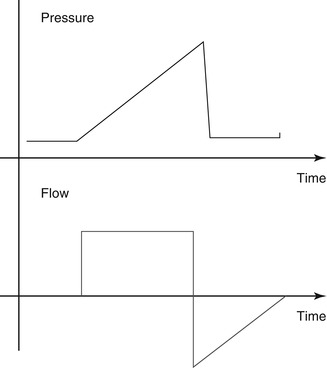

Fig. 8.4
Flow and pressure waveforms for constant-flow (volume-targeted) ventilation. Note the square configuration of the volume flow wave and the gradual increase (ramping) of pressure, creating a “shark’s fin” appearance
True volume cycling (where inspiration ends after the delivery of a specific volume of gas to the patient) is not yet feasible in the neonate. Because endotracheal tubes are uncuffed, there is almost always some degree of leak around the endotracheal tube. In addition, the ventilator must be able to measure the volume of gas at the proximal airway, not at the machine. Some of the gas leaving the machine will be compressed in the ventilator circuit, especially when the lungs are stiff. This is referred to as compressible volume loss and can be substantial.
Constant-flow (volume-targeted) ventilation was briefly popular in the late 1970s, but technological limitations precluded its widespread use, and it was largely abandoned until the development of microprocessor-based ventilation and small, lightweight, low dead-space transducers. Recent clinical evidence suggests that this might be a better way to ventilate preterm infants with respiratory distress syndrome. One feature of constant-flow ventilation is the auto-weaning of pressure. As compliance improves, less pressure is required to deliver the desired tidal volume, and the machine adjusts the peak inspiratory pressure instantaneously. Theoretically, this should be advantageous in avoiding both barotrauma (by weaning pressure) and volutrauma (by limiting volume). Indeed, early meta-analysis of volume vs pressure trials has shown a decreased incidence of air leak, a decreased duration of mechanical ventilation, and a strong trend toward decreased chronic lung disease. Recent trials are summarized in Table 8.3.
Table 8.3
Summary of key trials evaluating volume-targeted ventilation
Study | Randomization | Participants | Intervention | Outcome measures |
|---|---|---|---|---|
Cheema and Ahluwalia, (2001) | Randomized double crossover trial | 40 infants <34 weeks’ gestation requiring mechanical ventilation for RDS Exclusions: Infants requiring muscle relaxants or with lethal congenital anomalies | SIPPV alone vs SIPPV + VG, or SIMV alone vs SIMV + VG | Primary: Peak airway pressure Secondary: Mean airway pressure, expired tidal volume, minute volume, FiO2, and transcutaneous CO2 and O2 pressure |
D’Angio et al. (2005) | Randomized controlled trial | 213 infants who required mechanical ventilation and were at least 24 weeks’ gestational age and weighed 500–1,249 g at birth | PRVC vs TCPL SIMV | Primary: Proportion of infants who were alive and extubated at 14 days of age Secondary: Proportion of infants who were alive and extubated at 28 days of age or 36 weeks post-conceptual age, age at final extubation, mortality, failure of ventilatory mode, incidence of BPD, air leaks, pulmonary hemorrhage, PDA, IVH, PVL, NEC, ROP, home oxygen use, and numerous other parameters |
Herrera et al. (2002) | Randomized crossover trial | 17 infants between 600 and 1,200 g with respiratory failure Exclusions: Severe congenital anomalies, perinatal asphyxia, sepsis, symptomatic PDA, Grade 3–4 IVH, sedation, and clinical instability as defined by attending neonatologist | First nine infants: SIMV alone vs SIMV + VG (4.5 mL/kg) Next eight infants: SIMV alone vs SIMV + VG (4.5 mL/kg) vs SIMV + VG (3 mL/kg) | Peak inspiratory pressure, mean airway pressure, number of ventilator and patient generated inflation, tidal volume, FiO2, SPO2, and TcPO2 |
Keszler and Abubakar, (2004) | Randomized controlled trial | 18 infants <34 weeks’ gestation with RDS on mechanical ventilation Exclusions: Patients with congenital anomalies, receiving neuromuscular paralysis or narcotic agents, or with >30 % endotracheal tube leak | Assist control only vs assist control + VG | Primary: Percentage of time that tidal volume and PaCO2 were outside target range (4–6 mL/kg and 35–45 Torr, respectively) |
Lista et al. (2004) | Randomized controlled trial Stratified by treatment center and gestational age (25–28 weeks and 29–32 weeks) | 53 infants between 25 and 32 weeks’ gestational age, on mechanical ventilation for severe RDS Exclusions: Lethal anomalies, use of paralytic agents, IVH (Grade 3–4), sepsis, or suspected infection | PSV alone vs PSV + VG (5 mL/kg) | Primary: Concentrations of IL-6, IL-8, and TNF-ÿ in tracheal aspirates on days of life 1, 3, and 7 Secondary: Duration of ventilation, airway pressure, incidence and rate of treatment for PDA, number of surfactant doses, incidence of air leaks, IVH, PVL, ROP, oxygen dependency at 28 days and/or 36 weeks post-conceptual age, and survival |
McCallion and Morley (2005) | Meta-analysis of the following four trials: Keszler and Abubakar (2004) Lista et al. (2004) Piowtrowski et al. (1997) Sinha et al. (1997) | 178 infants <37 weeks’ gestation Exclusions: Lethal congenital anomalies, muscle relaxation, suspected sepsis, lack of arterial access, narcotic use, ETT leaks >30 %, severe IVH, asphyxia, pneumothorax, and meconium aspiration syndrome | Volume-targeted vs pressure-limited ventilation | Primary: Hospital mortality Death or need for supplemental oxygen at either 28 days of life or 36 weeks’ post-conceptual age Secondary: Failure of ventilatory mode or need for new use of muscle relaxants, duration of respiratory support, adverse blood gas measurements, PDA, air leaks, growth, IVH, PVL, neurodevelopmental outcome, need for supplemental oxygen at either 28 days of life or 36 weeks’ post-conceptual age among survivors, and impact of mode of volume-targeted ventilation |
Piowtrowski et al. (1997) | Randomized controlled trial | 60 infants with RDS or congenital pneumonia requiring mechanical ventilation and weighing <2,500 g Exclusions: Terminal state of infant at admission, air leaks, congenital anomalies, sepsis, and meconium aspiration | PRVC vs TCPL IMV | Primary: Duration of ventilation and incidence of BPD Secondary: Incidence of air leaks, IVH, hypotension, NEC, PDA, and need for sedation |
Singh et al. (2006) | Randomized controlled trial A priori stratification into two groups according to birth weight (600–1,000 g and 1,001–1,500 g) | 109 infants weighing between 600 and 1,500 g with gestational ages between 24 and 31 weeks, requiring mechanical ventilation and surfactant therapy Exclusions: Severe congenital malformations | VCV vs TCPLV Tidal volumes maintained between 4 and 6 mL/kg for both groups | Primary: Time from study entry until achievement of either an alveolar–arterial oxygen gradient <13 kPa (100 mmHg) or a mean airway pressure <8 cm H2O for at least 12 h Secondary: Duration of ventilation or respiratory support, survival to discharge, incidence of CLD, IVH, PVL, PDA, or NEC |
Singh et al. 2009 | Long-term outcomes from prior RCT (Singh et al. 2006) | 90 of the 109 infants in the 2006 study were followed Median corrected age at follow-up was 22 months | VCV vs TCPLV Patients prospectively followed with medical assessments and parental interviews via a structured questionnaire | Mortality, readmission rate, pulmonary outcomes (including the frequency of cough or wheeze and use of pulmonary medications), and gross neurodevelopmental outcome |
Sinha et al. (1997) | Randomized controlled trial | 50 preterm infants with RDS and birth weights of at least 1,200 g, requiring mechanical ventilation and surfactant therapy Exclusions: Pneumonia, sepsis, congenital malformations, lack of arterial access. | VCV vs TCPLV Tidal volumes maintained between 5 and 8 mL/kg for both groups | Primary: Time from study entry until achievement of either an alveolar–arterial oxygen gradient <13 kPa (100 mmHg) or a mean airway pressure <8 cm H2O for at least 12 h Secondary: Incidence of IVH, PVL, PDA, or BPD at 36 weeks post-conceptual age |
8.1.1.5.1 Conclusions
Neonatal mechanical ventilation has advanced dramatically over the past 10 years. The advent of microprocessor-based technology has revolutionized the concepts of ventilating newborns in respiratory failure. Strategies are now formulated based on the underlying pathophysiology and subsequently modified by the response of the patient and the interaction between the patient and the ventilator. Enhanced monitoring has improved patient safety. Long-term outcomes are still under investigation, but early information suggests that the future is indeed bright.
Essentials to Remember
Conventional ventilation refers to systems that deliver gas volumes that approach physiological tidal volumes.
High-frequency devices deliver gas volumes less than anatomical dead space.
Constant-flow ventilation is used to provide volume-targeted ventilation.
Variable-flow ventilation is used to provide pressure-controlled and pressure-support ventilation.
Monitoring systems are critical to patient safety and ventilator performance.
8.1.2 Patient–Ventilator Interface
Steven M. Donn15
(15)
Division of Neonatal-Perinatal Medicine, F4790 C.S. Mott Children’s Hospital, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5254, USA
8.1.2.1 Introduction
The interface refers to the way in which the ventilator circuit is connected to the patient. The interface is classified as invasive when the ventilator circuit attaches to a tube that is placed directly into the patient’s hypopharynx (endotracheal tube) or trachea (tracheostomy tube) for the delivery of positive-pressure ventilation. Noninvasive ventilation refers to positive-pressure ventilation that is delivered to the patient by a mask that covers the nose and mouth or by prongs or cannulas that are inserted into the nares. Systems that deliver negative-pressure ventilation may or may not use an interface with the airway (Chatburn 2003). This chapter will focus only upon invasive ventilation interfaces.
Educational Goals
Understand the concepts of the patient–ventilator interface.
Differentiate invasive and noninvasive ventilation.
Comprehend the effects of the patient circuit and endotracheal tube on mechanical ventilation.
8.1.2.2 Effects of the Patient Circuit
The patient circuit consists of tubing which conducts gas flow from the ventilator to the patient (inspiratory limb) and from the patient to the atmosphere (expiratory limb). The inspiratory limb may also pass through a heated source of humidification.
The ventilator circuit has its own characteristics regarding compliance and resistance. This impacts the actual volume of gas which reaches the patient. If the circuit is very flexible and is easily distorted and the compliance is greater than that of the patient, less gas will reach the patient compared to a more rigid circuit. On the other hand, if the circuit is very rigid and the patient’s lungs are very stiff, some of the gas within the circuit will be compressed and not reach the patient (Chatburn 2003). This is referred to as compressible volume loss and represents the difference between the volume of gas that leaves the ventilator and the volume of gas that actually reaches the patient airway.
Thus, the pressure, flow, and volume that actually reach the proximal airway are different from the settings that the clinician sets on the ventilator. A portion of this may result from errors in calibration or inaccuracy of measurement, but most relates to circuit compliance and resistance. Volume and flow leaving the ventilator is greater than that measured at the airway because of circuit compliance, whereas the pressure measured at the inspiratory side of the ventilator will be higher than that at the proximal airway because of circuit resistance (Chatburn 2003). Differences will also result from gas leaks anywhere in the circuit or connectors.
This is especially important during volume-targeted ventilation (see Sect. 8.1.3). Although the clinician orders a set volume of gas to be delivered to the patient, the actual volume reaching the proximal airway will be considerably less. For instance, even if a patient has reasonably compliant lungs and the circuit compliance is 0.5 mL/cm H2O, less than half of the delivered gas volume will reach the patient. For this reason, it is imperative that measurements of tidal volume be performed at the airway and not calculated from the machine, especially in small, preterm babies, where even a small variance can have a huge impact (Cannon et al. 2000) (Fig. 8.5).

Fig. 8.5
Preterm newborn infant receiving mechanical ventilation. Note the interface between the ventilator and the baby, consisting of ventilator circuit and its connection to the oral endotracheal tube
Because medical grade oxygen and air contain virtually no water, it is imperative that inspiratory gas be heated and fully humidified before delivery to the patient to avoid damage to the respiratory epithelium. Optimally, the temperature of the gas should be close to body temperature by the time it reaches the airway (Schulze 2006). Condensation (rainout) in the ventilator circuit can be troublesome. It may disrupt laminar flow, causing turbulence and ineffective gas exchange. It may also be the source of auto-cycling in patient-triggered ventilation (see Sect. 8.1.3). Humidification will also affect the viscosity of the delivered gas and thus contribute to circuit resistance.
8.1.2.2.1 The Endotracheal Tube
During invasive ventilation, the ventilator circuit is ultimately attached to an endotracheal or tracheostomy tube. In neonatal intensive care, the most commonly used endotracheal tubes range from 2.5- to 4.0-mm internal diameter. They are inserted from 7 to 10 cm, measured from the lip (for orotracheal tubes), depending upon the size of the baby. It should be remembered that flow through a tube is proportional to the fourth power of the radius and is related linearly to the length. Choosing the proper tube size is important. If the tube is too small, resistance will increase substantially (Oca et al. 2002), the tube will be more prone to become obstructed, and there may be a significant leak around the uncuffed endotracheal tubes used in newborns and small infants. Leaks may interfere with proper functioning of the ventilator and result in significant discrepancies between inspiratory and expiratory tidal volumes. If the leak exceeds the trigger threshold, auto-cycling may occur (see Sect. 8.1.3). Conversely, if the endotracheal tube is too large, damage may occur to the anatomical structures of the airway. The depth of insertion is also important. If too high, inadvertent extubation may occur and gas leak may be more prominent. If too low, right main bronchus intubation may occur, with subsequent atelectasis of the left lung and overdistension of the right lung.
Clinicians must also be aware that despite adequate external fixation at the lip, the endotracheal tube is still mobile within the trachea, and the location of its tip changes with respect to changes in position of the head and neck. When the head is flexed, the endotracheal tube tip will move deeper into the airway; when the head and neck are extended or laterally rotated, the tip will be withdrawn (Donn and Kuhns 1980). Although it seems intuitive that better fixation can be accomplished with a nasotracheal intubation, this is not the case (Donn and Blane 1985).
Endotracheal tube position can be ascertained using a disposable capnometer to detect exhaled carbon dioxide, followed by radiographic confirmation. The capnometer is temporarily attached to the endotracheal tube connector and undergoes a color change from purple to yellow when exposed to carbon dioxide (Aziz et al. (1999). Because of the aforementioned tube movement, radiographs should be obtained with the patient’s head and neck in a neutral position and in the midline (Donn and Kuhns 1980). Once verified in appropriate position, excess external length of the tube should be trimmed for the reasons cited in Table 8.4.
Table 8.4
Complications of long external endotracheal tube length
Increased dead space |
Increased resistance |
Less efficient gas exchange |
Increased work of breathing |
Higher risk of kinking (obstruction) |
Greater risk of inadvertent and self-extubation |
Increased risk of infection (pooling of secretions) |
More difficult to suction |
Increased risk of auto-cycling |
8.1.2.3 Apparatus Dead Space
Dead space classically refers to parts of the respiratory system that do not participate in gas exchange. Within the lung, gas exchange only occurs in the alveoli and terminal portions of the smallest airways. Gas exchange does not take place in the conducting airways, and this is often referred to as anatomical dead space. If, within the lung, there are areas of underperfused alveoli that are not participating in gas exchange, they are referred to as alveolar dead space. The anatomical and alveolar dead-space volumes are collectively referred to as total or physiological dead space. Wasted ventilation, the proportion of tidal gas that is delivered to the patient but not utilized in gas exchange, is defined by the ratio of dead-space volume to tidal volume (Chatburn 2003).
In this regard, the ventilator circuit and any attached apparatus can be thought of as an extension of the anatomical dead space, since not all of the gas flowing through it is involved in pulmonary gas exchange. This has also been referred to as mechanical dead space. Unless a way can be found to compensate for the increase in dead space, the imposed work of breathing will also increase.
Several devices, primarily used for monitoring, can now be added to the ventilator circuit but at the cost of additional dead space (Table 8.5). These include external transducers to measure flow, volume, or pressure; capnometers to measure end-tidal carbon dioxide; and stand-alone pulmonary function or mechanics devices, which are capable of measuring multiple parameters, including volumetric carbon dioxide (enabling the calculation of dead space to alveolar ventilation ratios). There are two types of capnometers: mainstream and sidestream. The mainstream capnometer may add considerable dead space, depending on the model, whereas sidestream capnometers do not. However, the risk of dilution by expired gas through entrainment of ambient air may adversely affect measurements (Sinha and Donn 2006a). Many centers prefer to use closed system suctioning devices, which are placed in line and add dead space to the circuit. Hygroscopic heat and moisture exchangers are sometimes used in place of a heated humidification system and can also add appreciable dead space (Schulze 2006).
Table 8.5
Devices which add mechanical dead space
Flow, pressure, or volume transducers |
Capnometers |
Stand-alone pulmonary function or mechanics devices |
Closed suctioning devices |
Hygroscopic heat and moisture exchangers |
8.1.2.4 Imposed Work of Breathing and Pressure-Support Ventilation
The imposed work of breathing refers to the amount of work needed to overcome the collective effects of the endotracheal tube, ventilator circuit, and demand valve, if a demand system is used (Sinha and Donn 2006a). It may be thought of as the “tax” a patient must pay for receiving mechanical ventilation. The age-old adage of “breathing through a straw” is a suitable analogy for the imposed work of breathing.
The imposed work of breathing becomes more significant the more a patient breathes spontaneously. Spontaneous inflation must be supported in some way to overcome the imposed work of breathing. During most forms of mandatory mechanical ventilation, spontaneous inflation are supported only by PEEP, and thus, it is not hard to understand why weaning the ventilator rate and shifting the burden of respiratory work to the patient often fails as a weaning technique (Sinha and Donn 2006b).
Pressure-support ventilation (PSV) was developed to assist spontaneous breathing by overcoming the imposed work of breathing. It is an inspiratory pressure assist applied to spontaneous inflation. It is patient triggered, flow cycled, and pressure limited (see Sect. 8.1.3). Thus, the patient controls its onset by triggering it, its duration of inspiration (by flow cycling), and its frequency. The clinician sets the pressure limit (and thus controls the degree to which the breath is supported) and an inspiratory time limit, which the patient may not exceed. It may be used in conjunction with synchronized intermittent mandatory ventilation (SIMV) or alone, if the patient has reliable respiratory drive. If the pressure is set high enough to provide a full tidal volume breath, the level of support is described as PSmax; if the level is just enough to overcome the imposed work of breathing, it is described as PSmin (Sinha and Donn 2006a). The best estimate of PSmin in the newborn is the pressure required to deliver a tidal volume of 3–4 mL/kg (Fig. 8.6).
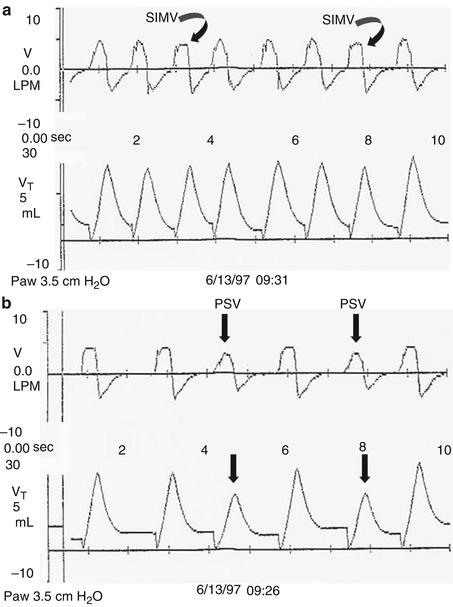

Fig. 8.6
Pressure support ventilation. Real-time pulmonary graphics showing flow and volume waveforms. (a) Patient is receiving PSmax. Note that the tidal volumes received during both the volume (square wave) SIMV inflation (arrows) and the pressure-support inflation are the same. (b) The patient is receiving pressure support to partially support spontaneous inflation (PSV). Note that the tidal volume delivery is less than that delivered during SIMV
8.1.2.5 Summary
The patient–ventilator interface and ventilator circuit play important roles in determining the success of assisted mechanical ventilation. Careful attention must be paid to selecting the proper interface for invasive ventilation and to assuring its optimal location and fixation. The ventilator circuit and various devices, which can be added to it, such as capnometers and closed suctioning systems, also add mechanical dead space and increase the imposed work of breathing. Clinicians must find ways to compensate for this, by either adding more flow or volume or assisting spontaneous breathing by adding pressure support to spontaneous inflation.
Essentials to Remember
The patient–ventilator interface and ventilator circuit are key elements of mechanical ventilation.
Components added to the ventilator circuit add dead space and increase the imposed work of breathing.
PSV is a novel way to help overcome the imposed work of breathing.
8.1.3 Ventilator Modes
Steven M. Donn16 and Sunil K. Sinha17
(16)
Division of Neonatal-Perinatal Medicine, F4790 C.S. Mott Children’s Hospital, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5254, USA
(17)
Paediatrics and Neonatal Medicine, The James Cook University of Hospital, University of Durham, Middlesbrough, UK
8.1.3.1 Introduction
Ventilator modes refer to the specific patterns of spontaneous and mandatory mechanical inflation. Spontaneous inflation are those initiated by the patient. They may or may not result in a mechanical breath, depending upon whether or not the patient is receiving triggered ventilation. Mandatory inflation are initiated by the ventilator based on an initiating factor, usually time.
Controlled ventilatory modes include intermittent mandatory ventilation (IMV), synchronized intermittent mandatory ventilation (SIMV), and assist/control ventilation (A/C). In spontaneous ventilatory mode, pressure support ventilation (PSV) involves mechanical support applied only to spontaneous inflation, which may be combined with SIMV or used as a singular mode (Sinha and Donn 1996; Donn and Sinha 2001).
Educational Goals
Understand the specific patterns of spontaneous and mandatory mechanical inflation.
Recognize the three component waveforms: pressure, volume, and flow.
Differentiate phase and control variables, and understand trigger variables.
8.1.3.2 Controlled Ventilation
8.1.3.2.1 Waveforms
The three airway signals, pressure, volume, and flow, may be plotted against time to produce graphic waveforms. An understanding of the waveforms is essential to comprehending how controlled ventilation works (Donn 1997; Sinha et al. 1996; Bhutani 2002).
The pressure waveform displays changes in airway pressure over time. If positive end-expiratory pressure is utilized, the baseline pressure will serve as the starting point for inspiration. As airway pressure increases during inspiration, the waveform will increase until it reaches its highest value, referred to as the peak inspiratory pressure (PIP). Pressure subsequently declines until it reaches the end-expiratory level. The area under the curve represents the mean airway pressure. Since oxygenation is a function of mean airway pressure, ventilatory maneuvers which increase the area under the curve may improve oxygenation. These include raising the PEEP, increasing the PIP, lengthening the inspiratory time, and to a lesser extent, increasing the rate.
The volume waveform looks similar to the pressure waveform, except that in the ideal situation, the waveform should reach the zero baseline at end expiration. Failure to do so indicates the presence of a volume leak around the endotracheal tube. The volume waveform peaks earlier in inspiration when pressure is controlled in contrast to the situation in which volume or flow is controlled, where the slope will be less.
The flow waveform differs from both the pressure and volume waveforms by having components that are both above the baseline (inspiration) and below the baseline (expiration). In other words, positive flow represents gas delivered into the airway, and negative flow represents gas egressing from the airway. As inspiration commences, there is a rapid flow of gas into the airway producing a sharp upswing in inspiratory flow, referred to as accelerating inspiratory flow. At its most positive level, this is referred to as peak inspiratory flow. Inspiratory flow then decelerates. However, note that this is still a positive value, thus airflow is still inspiratory although slower, and this component is referred to as decelerating inspiratory flow. Between the end of inspiration and the start of expiration, the flow waveform reaches a zero flow state (baseline) (An exception to this occurs during flow cycling discussed below). As expiration begins, there is an acceleration of expiratory flow, the sharp downward deflection below baseline, which at is most negative value is referred to as peak expiratory flow. Following this, expiratory flow decelerates, and although this scalar is in an upward direction, it is still negative and represents decelerating expiratory flow.
8.1.3.2.2 Control Variables
A control variable is the primary variable that the ventilator utilizes to produce the inspiratory phase of a mechanical breath. According to the equation of motion, there are three possible variables that can be controlled: pressure, volume, or flow. However, only one of these can be directly controlled at a time (Carlo et al. 2006; Chatburn 1995).
If pressure is the control variable, the pressure waveform will remain constant, even if there are changes in lung mechanics (compliance and resistance), and flow and volume will be variable. Positive-pressure ventilators control airway pressure, whereas negative pressure ventilators control body surface pressure.
If volume is the control variable, both the volume and flow waveforms will remain constant, and changes in lung mechanics will result in variability in airway pressure. True volume controllers must measure volume and use this measurement to control volume delivery. Volume may be controlled directly, using a device such as a piston or bellows, or indirectly by controlling airway flow (flow is defined as the time rate of volume delivery). Thus, true volume control is not technically possible in neonatal ventilation because cuffed endotracheal tubes are not used, and there is almost always some degree of volume leak around the endotracheal tube. For this reason, it is more appropriate to refer to this type of ventilation as volume-targeted or volume-limited (Sinha and Donn 2001).
If flow is the control variable, again both the volume and flow waveforms will remain constant, with pressure varying as lung mechanics change. The means for controlling flow include simple flow meters or more technical proportional solenoid valves.
8.1.3.2.3 Phase Variables
Each breath, whether spontaneous or mechanical, consists of four phases: the initiation of inspiration, inspiration itself, the end of inspiration, and expiration. Phase variables refer to parameters that are measured and utilized to initiate, sustain, or terminate some phase of the ventilatory cycle. They consist of a trigger variable that initiates inspiration, a limit variable that restricts the magnitude of some parameter (i.e., pressure) during inspiration but which does not terminate inspiration, and a cycle variable that causes inspiration to end. Positive end-expiratory pressure is sometimes referred to as the baseline variable (Carlo et al. 2006).
8.1.3.2.3.1 Trigger Variables
A mechanical breath may start in response to a spontaneous breath (patient-triggered breath) or it may be initiated by the ventilator (mandatory or control breath). Patient-triggered inflation are initiated by a signal derived from the patient, which represents spontaneous respiratory activity, such as a change in airway pressure or flow. In the absence of a trigger mechanism, time is used as a trigger, where a mechanical breath is provided at intervals chosen by the clinician (Hird and Greenough 1991a; Hummler et al. 1996).
8.1.3.2.3.2 Limit Variables
Typically, limit variables restrict or maintain a parameter within the limits preset by the clinician. Pressure, flow, or volume can all be used as limit variables. An important distinction is that the limit variables are applied during inspiration but do not end it. It is a misnomer to consider time as a limit variable, because it actually ends inspiration and is thus a cycle variable.
8.1.3.2.3.3 Cycle Variables
When some variable reaches a preselected level, the inspiratory phase ends and the breath is cycled into expiration. The cycle variable refers to this measured variable used to end inspiration.
Time has been the most common cycle variable. The clinician chooses an inspiratory time limit, and the inspiratory phase of the breath is terminated when this time elapses. Some devices allow the clinician to prolong inspiration through the use of an inspiratory hold. Inspiratory gas flow occurs during the inspiratory flow time, but during the inspiratory hold time, the exhalation valve remains closed, but there is no inspiratory gas flow. Here, the inspiratory time will be the sum of the inspiratory flow time and the inspiratory hold time. Time cycling is used as a “backup” mechanism during assist/control and pressure support ventilation, where flow is the primary cycle variable (see below). Phase variables refer to parameters that are measured and utilized to initiate or terminate some phase of the ventilatory cycle, e.g., cycling mechanisms.
Pressure cycling is used primarily for alarms. During pressure cycling, inspiratory flow is delivered until the preset pressure level is attained. Inspiratory flow then ceases and expiratory flow begins.
Volume cycling delivers inspiratory flow until a preset volume of gas has been delivered to the airway, after which inspiratory flow stops and expiratory flow begins. Some volume-cycled devices allow the clinician to maintain inspiration beyond this point by using an inspiratory hold, but in this case the cycle variable is time. An important distinction must be made between the volume of gas which leaves the ventilator and the actual volume that reaches the patient. These are not the same because of compression of gas within the ventilator circuit. Even if delivered volume is measured at the proximal airway, true volume cycling cannot be accomplished without a cuffed endotracheal tube because of gas leaks around an uncuffed tube (Sinha and Donn 2001; Hird and Greenough 1991a).
Flow cycling is a technique used to terminate inspiration when decelerating inspiratory flow has declined to a certain percentage of peak inspiratory flow. During flow cycling, inspiration cycles directly into expiration at this point, and there is only an instantaneous zero flow state between inspiration and expiration. Flow cycling allows the patient to control the duration of inspiration and thus improve patient–ventilator synchrony by adding expiratory synchrony, often called an expiratory trigger (see below). Flow cycling also enhances patient safety. Because inspiration is terminated as a percentage of peak flow, the risks of inversion of the inspiratory-to-expiratory ratio (I:E), gas trapping, and inadvertent PEEP during patient-triggered ventilation (if the patient becomes tachypneic) are considerably less than with time cycling. During flow cycling, the actual inspiratory time will be less than the set inspiratory time when inflation are terminated by the flow change (Prinainak et al. 2003).
8.1.3.2.4 Controlled Modes of Ventilation
8.1.3.2.4.1 Intermittent Mandatory Ventilation
The original mode of mechanical ventilation was IMV. In this mode, the clinician chooses a set rate at which mechanical inflation will be delivered to the patient, and the ventilator will deliver the inflation at regular intervals. In between the mechanical inflation, the patient may breathe spontaneously. However, the spontaneous and mechanical inflation have no fixed relationship to one another and function independently. This may lead to asynchronous breathing and significant variability in delivered gas volumes. For instance, if the patient initiates a spontaneous breath while a mechanical breath is also in the inspiratory phase, the delivered gas volume will be considerably larger than in the situation where the patient is actively attempting to exhale against an incoming mechanical breath. This is frequently described as “fighting the ventilator.”
Asynchrony has been shown to produce numerous problems, including inefficient gas exchange, increased work of breathing, gas trapping and a higher incidence of thoracic air leaks (Greenough and Morley 1984), and irregular arterial blood pressure and cerebral blood flow velocity patterns. The latter have been associated with the development of intraventricular hemorrhage in preterm newborns with respiratory distress syndrome (Perlman et al. 1985).
Infants managed with IMV are frequently weaned from ventilatory support by reducing the IMV rate. This needs to be done cautiously. If done too rapidly, the baby may become increasingly fatigued during the weaning process and may not tolerate extubation.
8.1.3.2.4.2 Synchronized Intermittent Mandatory Ventilation
In SIMV, the clinician also sets a rate at which the mandatory inflation will be delivered, and the patient may also breathe spontaneously between the mandatory inflation. However, the ventilator attempts to synchronize the onset of the inspiratory phase of the mechanical breath to the onset of a spontaneous breath if one occurs within a timing window (Donn and Becker 2003). For example, if the SIMV rate is set at 30 inflation/min, a mandatory breath will be delivered approximately every 2 s. When it is time to deliver that breath, the ventilator will respond to the start of a spontaneous breath that occurs shortly before or shortly after that point. If no patient effort is detected within the timing window, a mechanical breath will be provided. Thus, SIMV removes much of the inspiratory asynchrony of IMV, but if the set inspiratory time is longer than the patient’s own inspiratory time, expiratory asynchrony will still occur. This can be alleviated by using flow cycling.
As with IMV, spontaneous breathing between mandatory inflation is supported only by the baseline pressure (PEEP). Because babies have intrinsically high respiratory rates, both IMV and SIMV provide a higher work of breathing for the baby compared to A/C, because a large proportion of inflation are insufficiently supported. Use of either A/C or PSV (alone or in combination with SIMV) can overcome this problem. Problems similar to those for IMV also occur during weaning from SIMV.
8.1.3.2.4.3 Assist/Control Ventilation
During A/C, inflation initiated by the patient are “assisted” by the mechanical breath, while those that occur as mandatory inflation are “controlled.” Assist/control ventilation is accomplished by utilizing a trigger variable to respond to patient effort. In the newborn, this is most commonly derived from a change in airway flow (see below). If the spontaneous effort exceeds the trigger threshold, the ventilator will respond by delivering a mechanical breath, which has a limit and cycling variable chosen by the clinician. If the patient fails to breathe or if the breath effort is insufficient to reach the trigger threshold, a control breath will be delivered at a rate set by the clinician. Thus, every patient breath that meets the trigger threshold will result in the delivery of a synchronized mechanical breath. The level of support during the assisted breath will be the same as during a control breath and is determined by the limit variables. For instance, inflation may be fully supported and deliver a full tidal volume or they may be partially supported (by adjusting pressure or volume) (Donn and Becker 2003; Greenough and Pool 1988).
Weaning during A/C is different from IMV or SIMV. As long as the patient is breathing above the control rate, further reductions in the rate will have no effect on the mechanical ventilatory rate (Sinha and Donn 2002). The primary weaning strategy during A/C is a reduction in pressure or volume. Some clinicians will extubate directly from A/C, while others prefer to switch to SIMV/PS during the weaning stage of illness.
Combining A/C with flow cycling can achieve complete synchrony between the baby and the ventilator. Inflation are initiated by spontaneous patient effort and they are likewise terminated in very close proximity to the end of the spontaneous inspiratory phase. Every breath is virtually identical, and numerous short-term physiological advantages have been demonstrated for A/C compared to either IMV or SIMV (Donn et al. 1994; Donn and Sinha 1998).
8.1.3.2.5 Synchronization Principles and Trigger Systems
8.1.3.2.5.1 Introduction
The concepts of patient-triggered and synchronized ventilation were practiced in adult and even pediatric respiratory care long before they became available to neonatal patients. Technological limitations precluded the ability to derive appropriate trigger signals and monitoring systems to safely accomplish synchronized ventilation until the decade of the 1990s.
As described above, one of the major problems with mechanical ventilation is asynchrony between the patient and the machine. Asynchrony results not only inefficient gas exchange but also contributes to respiratory and neurologic morbidity and increased cost of care. Until the advent of patient-triggered ventilation, clinicians had limited options to deal with asynchrony. Ventilator settings could be increased to try to “capture” or “overbreathe” the patient, but this was at the risk of increasing ventilator-induced lung injury. Patients could be sedated, but this often depressed the respiratory drive and prolonged the duration of mechanical ventilation. In severe cases, skeletal muscle relaxants could be used, but long-term administration resulted in numerous problems, including muscle atrophy, edema, and ventilator dependence.
8.1.3.2.5.2 Principles of Synchronization
Synchronized ventilation is an attempt to match spontaneous and mechanical breathing as closely as possible. It allows the patient to have control over some ventilator variables that were previously set by the clinician and which overrode the patient’s own breathing contributing to asynchrony.
The primary principle of synchronized ventilation is the use of a marker or surrogate of spontaneous breathing as a mechanism to trigger the delivery of a mechanical breath in as close proximity as possible to the spontaneous breath and to mimic the patient’s own pattern of breathing. Ideally, this should occur for both the onset of inspiration and the termination of inspiration. Thus, it should include both the trigger and cycle variables in its design (Greenough and Pool 1988).
There are additional concepts that are important to the performance of patient-triggered ventilation. First, the trigger signal needs to be a reliable indicator of spontaneous breathing and not an artifact resulting from movement of nonrespiratory musculature. Second, the trigger sensitivity has to be appropriate for the patient; if it is too difficult to achieve, the work of breathing will be higher, as spontaneous inflation will not be supported beyond the baseline pressure, and if it is too sensitive, auto-cycling may occur (see below). Third, there needs to be a very short system response time or trigger delay. This time refers to the interval between reaching the trigger sensitivity and the rise in pressure at the proximal airway. If the trigger delay is too long, the patient may be nearly finished with the spontaneous inspiratory phase before help from the ventilator arrives. Finally, there should be minimal auto-cycling (false triggering) (Donn and Sinha 1998; Hird and Greenough 1990; Donn et al. 2000).
8.1.3.2.5.3 Trigger Systems
Various trigger systems were introduced into neonatal practice during the 1990s. As technological refinements occurred, several of these were replaced by systems with better sensitivity, shorter trigger delays, and less auto-cycling. In general, most systems now have trigger delays under 50 ms and work well on even the smallest patients (Laureen and Ronald 2000; Servant et al. 1992).
8.1.3.2.5.4 Abdominal Motion
One of the first trigger systems utilized in neonatal patients used a signal derived from abdominal motion during breathing (Hummler et al. 1996). An applanation transducer, such as the Graseby capsule, was placed on the abdomen and used to trigger a mechanical breath in response to spontaneous breathing. It appeared to work best in patients >2,000 g, but sensor placement was critical, and artifacts such as hiccups produced mechanical inflation unrelated to spontaneous breathing. In addition, there was only a single sensitivity setting, and tidal volume could not be measured. This technique has been largely abandoned.
8.1.3.2.5.5 Thoracic Impedance
Another early trigger system utilized changes in thoracic impedance, determined by standard electrodes used for electronic cardiorespiratory monitoring, to trigger inflation. In turn, the inspiratory cycle was terminated by active expiration. It, too, was dependent upon proper lead placement and maintenance of contact gel beneath them. It was also unable to measure tidal volume (Ferguson 2006).
8.1.3.2.5.6 Airway Pressure
Changes in airway pressure are also utilized to trigger mechanical inflation. As the patient begins to breath, there is a slight reduction in airway pressure, which serves as the marker of a spontaneous breath. Proper setting of the sensitivity level is a key. Compared to flow-triggered systems, pressure triggering requires more patient effort and is less suitable for the smaller babies. This system is relatively easy to use and can provide data from numerous airway metrics (Hird and Greenough 1990; Laureen and Ronald 2000).
8.1.3.2.5.7 Airway Flow
The most popular method of providing neonatal patient-triggered ventilation involves the use of a signal derived from changes in airway flow (Laureen and Ronald 2000; Hird and Greenough 1991a). As the patient initiates a spontaneous breath, there is a slight acceleration of flow at the proximal airway. This can be detected by one of the two transduction methods. The first of these uses a pneumotachograph (a variable orifice, differential flow transducer). In the center of the transducer, there is a membrane, which is distorted proportional to the amount of flow. This signal is used to trigger the ventilator, and it can be integrated to provide volume measurements. The second of these uses a heated wire anemometer. As gas flows over it, it is cooled, and the amount of current needed to return the wire to baseline temperature can be converted to a flow and volume signal.
Flow transducers are extremely sensitive, detecting flow changes as small as 0.1–0.2 L/min. This is about the amount of flow necessary to propel a dust ball. They are ideally suited for the tiny patients in the neonatal intensive care unit. The downside of flow transducers is the higher tendency for auto-cycling described below.
8.1.3.2.5.8 Neural Triggering
Another means of triggering mechanical ventilation is the utilization of neural impulses. One such system uses a neurally adjusted ventilatory assist technology in which a signal is derived when the vagus nerve stimulates the diaphragm. The electrical activity of the diaphragm is captured, transmitted to the ventilator, and used to assist the patient’s breathing. Both the ventilator and the diaphragm work with the same signal, minimizing trigger delay and theoretically reducing the risk of auto-cycling (see Sect. 8.1.3.3.4). There has been limited investigation of the technique in newborns, but it is an attractive hypothesis (Bernstein et al. 1995).
8.1.3.2.5.9 Auto-cycling
Auto-cycling is a phenomenon of patient-triggered ventilation. It occurs when something other than the patient’s effort triggers the ventilator in a repetitive fashion, often producing a string of rapid, identical inflation on graphic monitoring.
Flow-triggered systems have the highest incidence of auto-cycling, most likely as a consequence of their extreme sensitivity. Auto-cycling most commonly occurs because of leaks, either in the ventilator circuit or around the endotracheal tube. If the leak exceeds the trigger threshold, the trigger system will interpret this as patient effort and provides a mechanical breath. If the leak persists, another breath is provided, and so on. Auto-cycling can also occur if there is excessive condensation in the ventilator circuit, as the oscillating water can create a flow change that exceeds the trigger sensitivity.
Clinicians must learn to recognize auto-cycling and distinguish it from simple tachypnea. The major graphic feature of auto-cycling is its regularity and similarity of all the inflation. Tachypnea usually shows some degree of breath-to-breath variability in both rate and configuration.
There are several ways to deal with auto-cycling. First, try to eliminate any source of leaks in the circuit or equipment and be sure that there is no condensation in the ventilator circuit. Second, if it appears that the leak is originating from the airway, decreasing the trigger sensitivity may solve the problem. Some devices enable a measurement of the leak, and setting the sensitivity at a level that is slightly greater than this value can “fool” the ventilator and cease the auto-cycling (Donn and Becker 2003; Donn and Sinha 1998).
It is also important to realize that leaks may play havoc with flow cycling. If the leak is significant, the decline in the decelerating inspiratory flow waveform may not reach the termination point. This problem is eliminated by employing effective variable leak compensation in certain ventilators specifically designed for newborns with uncuffed ETT.
8.1.3.2.5.10 Clinical Evidence
Synchronized ventilation, whether provided by SIMV or A/C is clearly superior to IMV (Bernstein et al. 1994, 1996; Chan and Greenough 1994; Greenough et al. 2008). Virtually, every clinical trial has demonstrated short-term physiological benefit, including a shorter duration of ventilation, decreased thoracic air leaks, need for less sedation, and reduced hospital costs. Although there is a trend towards less chronic lung disease, it has been difficult to demonstrate this, as studies have been small and underpowered. It may also be affected by changing demographics, as smaller and more premature babies are now surviving. Nevertheless, IMV should be a mode of the past.
Essentials to Remember
Modes of ventilation include IMV, SIMV, A/C, and PSV.
The control variable is the primary variable utilized to produce the inspiratory phase of a mechanical breath.
The phase variables refer to parameters that are measured and utilized to initiate, sustain, or terminate some phase of the ventilatory cycle.
Synchronized ventilation is advantageous compared to IMV.
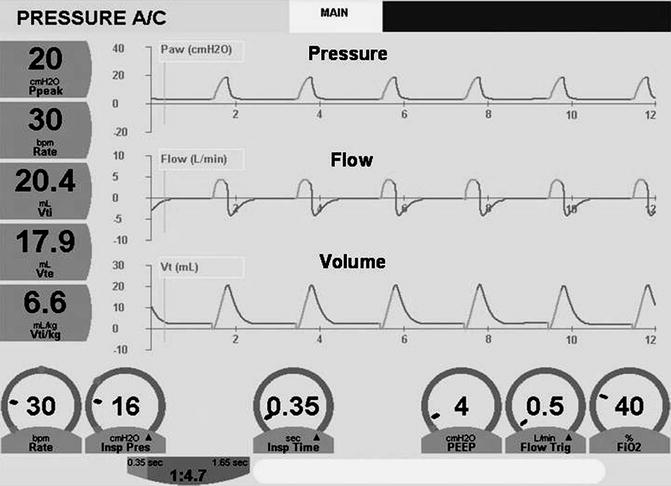
Fig. 8.7
Graphic waveforms. Pressure and volume (top and bottom) are similar and are always above the baseline. Flow waveform (middle) has two components. Inspiration is above the baseline and represents flow going into the patient (positive). This has accelerating (upward) and decelerating (downward) phases. Expiration is below the baseline and represents flow coming from the patient (negative). It also has accelerating (downward) and decelerating (upward) phases
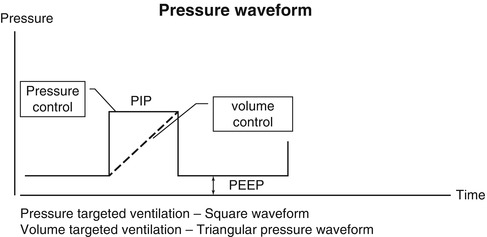
Fig. 8.8
Pressure waveform. Solid line represents pressure-targeted ventilation, which produces a square waveform. Dotted line represents volume-targeted ventilation, which produces a triangular or “shark’s fin” pressure waveform. Note that the expiratory portion of the waveform is above the baseline representing positive end-expiratory pressure (PEEP). The area under the curve, bounded by the peak inspiratory pressure (PIP) and PEEP, is the mean airway pressure
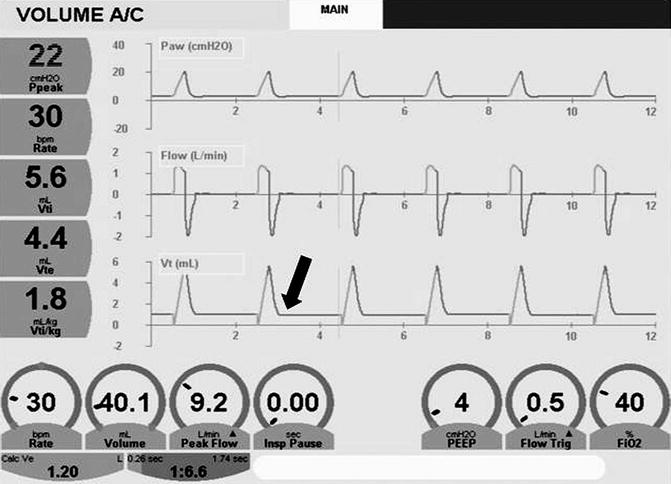
Fig. 8.9
The volume waveform fails to reach the baseline (arrow) because of a large endotracheal tube leak. Note discrepancy between the inspiratory (V ti) and expiratory (V te) tidal volumes
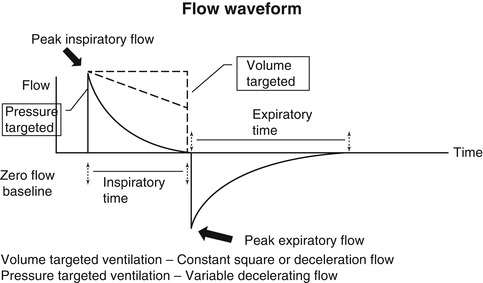
Fig. 8.10
Anatomy of the flow waveform. See text for description
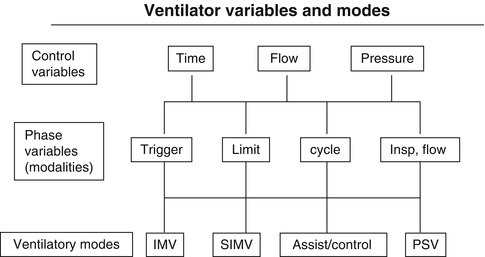
Fig. 8.11
Hierarchical classification of ventilator variables and modes. Although pressure support ventilation (PSV) is shown, it is a spontaneous and not a controlled mode
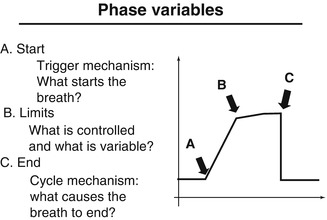
Fig. 8.12
Schematic diagram of a single ventilatory cycle noting points at which phase variables act
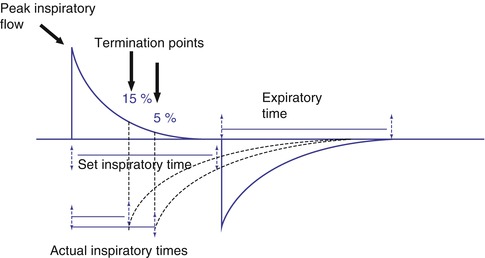
Fig. 8.13
Time versus flow cycling. When time is used to cycle a mechanical breath, inspiration ends when the preset limit has been reached. This may produce a prolonged zero flow state at the end of inspiration. When flow is used to cycle the breath, inspiration ends at a preselected termination point as a percentage of peak inspiratory flow. The actual inspiratory time is less than the set inspiratory time and is controlled by the patient
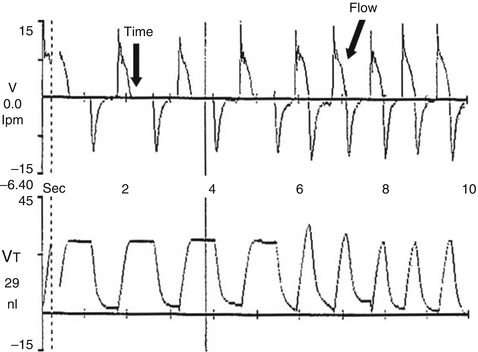
Fig. 8.14
Graphic display of the differences between time and flow cycling. The first four inflation are time cycled. Note the prolonged zero flow state at the end of inspiration (time arrow), which produces a plateau of volume delivery. The last five inflation are flow cycled. Note how the decelerating inspiratory flow waveform transitions immediately into expiration at the termination point (flow arrow) producing a spiked volume delivery waveform
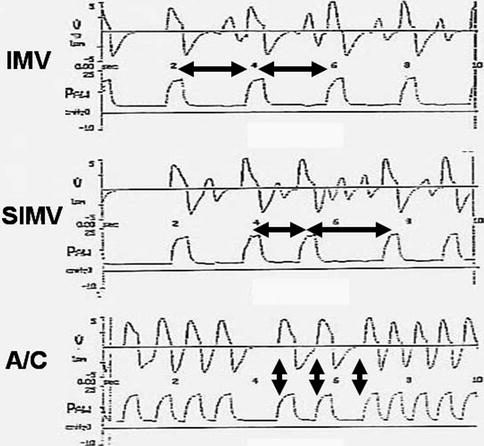
Fig. 8.15
Comparison of flow and pressure waveforms for intermittent mandatory ventilation (IMV), synchronized intermittent mandatory ventilation (SIMV), and assist/control ventilation (A/C). In IMV, mechanical inflation are delivered at regular intervals in accordance with the rate chosen by the clinician. Here, a breath is given every 2 s (note the intervals between the horizontal arrows), and the patient breathes spontaneously in between these inflation supported only by PEEP. In SIMV, the ventilator has a “timing window.” If the patient makes a detectable spontaneous effort, the ventilator will respond with a mechanical breath that is synchronized to the onset of the spontaneous breath. If the patient fails to breathe, a mandatory breath is provided. Note how this produces an irregularity in the breath rate (differences in the horizontal arrows). Again, the patient may breathe between the mechanical inflation supported only by PEEP. In A/C, each spontaneous breath that meets the trigger sensitivity results in a synchronously delivered mechanical breath. If flow cycling is also used, complete patient–ventilator synchrony can be achieved. Note the complete relationship of the flow and volume waveforms (vertical arrows), whether the patient breathes at a slow or fast rate
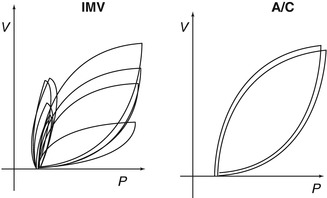
Fig. 8.16
Comparison of IMV and A/C. Note the variability in tidal volume delivery with IMV. Despite the fact that each breath reaches the same peak pressure, asynchrony results in wide differences in tidal volumes. During A/C, every breath is identical
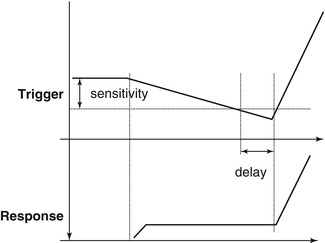
Fig. 8.17
Graphic representation of patient-triggered ventilation. The trigger responds when the patient meets the trigger sensitivity resulting in the delivery of a mechanical breath. The system response time, or trigger delay, is the interval between the trigger event and the rise in pressure at the proximal airway
8.1.3.3 Assisted Ventilation
Katherine C. Clement18 , Mark J. Heulitt18, Andreas Schulze19, Eduardo Bancalari20 , Jean-Michel Arnal21 and Guillaume Emeriaud22
(18)
Department of Pediatrics, University of North Carolina, 214 MacNider, CB 7221, Chapel Hill, NC 27599, USA
(19)
Department of Pediatrics, Alpert Medical School of Brown University, Women and Infants Hospital, 101 Dudley Street, Providence, RI 02905, USA
(20)
Division of Neonatology, Department of Pediatrics, University of Miami Miller School of Medicine, Miami, FL, USA
(21)
Service de réanimation polyvalente, Hopital Sainte Musse, 54 avenue Henry Sainte Claire Deville, Toulon, 83100, France
(22)
Soins Intensifs Pédiatriques, CHU Sainte-Justine, 3175, Côte Sainte-Catherine, Montréal, H3T 1C5, Canada
8.1.3.3.1 Pressure-Support Ventilation
Katherine C. Clement and Mark J. Heulitt
Education Aims
Understand the characteristics of a pressure-support breath.
Understand the three major phases of PSV.
Understand the physiological effects on the breathing pattern, oxygenation and ventilation, and work of breathing.
Understand the types of asynchrony during PSV and how it can be identified.
8.1.3.3.1.1 Introduction
Pressure-support ventilation (PSV) is a support mode used for spontaneously breathing patients. It augments inflation initiated by the patient which may improve patient–ventilator synchrony, reduce sedation needs, prevent disuse atrophy of respiratory muscles, and facilitate weaning (Brochard and Lellouche 2006; Sassoon et al. 2004). PSV may also improve work of breathing (Kornecki and Kavanagh 2007) and improve patient comfort (MacIntyre 1986; Thille et al. 2008).
8.1.3.3.1.2 Definition
PSV is a patient-triggered, pressure-limited, and flow-cycled form of mechanical ventilation used in patients with intact respiratory drive (Kornecki and Kavanagh 2007). A preset amount of positive pressure is delivered by the ventilator in synchrony with patient effort to raise airway pressure to a certain level (Brochard and Lellouche 2006). This preset pressure is known as the “pressure-support level” (Brochard and Lellouche 2006). A patient must generate a minimum negative inspiratory force that exceeds the preset ventilator flow or pressure sensitivity in order to trigger a breath from the ventilator (Kornecki and Kavanagh 2007). The set level of pressure support is then delivered and sustained until the ventilator senses the end of expiration, which is ideally a reflection of the end of patient demand (Brochard and Lellouche 2006). When inspiratory flow drops below a set threshold, which may be suggestive of relaxation of inspiratory muscles, the ventilator will cycle to the expiratory phase, open the expiratory valve, and release the pressure support (Brochard and Lellouche 2006). With this mode of ventilation, patients are able to control their own rate, inspiratory time, and tidal volume (Kornecki and Kavanagh 2007). There are no mandatory inflation delivered; however, there is a safety feature on most modern ventilators in the case of apnea where the ventilator will automatically shift into a control mode (Brochard and Lellouche 2006).
The three major phases of PSV (initiation, pressurization, and cycling off) may vary among ventilators, may be altered by patient effort, and/or may be adjusted by the clinician in some circumstances. Patients must trigger the ventilator with their own active effort. Trigger sensitivity may be adjusted to improve a patient’s ability to initiate a breath. Trigger delay is the time between the start of patient effort and the start of ventilator pressurization of the breath. Most ventilators respond in less than 100 ms, but there is some variability among ventilator models (Brochard and Lellouche 2006). The rate of pressurization also varies among ventilator models, but many allow for clinician adjustment. A regulatory mechanism should ensure the appropriate flow reaches the set pressure-support level and keeps the pressure constant until expiration occurs (Brochard and Lellouche 2006). A high speed of pressurization will produce a square pressure wave, whereas a lower speed of pressurization will attenuate this square shape (Iotta et al. 1991). Cycling off usually occurs when inspiratory flow falls below a specific threshold. This threshold can be changed on many ventilators (Brochard and Lellouche 2006). Sensing a small change in pressure above the set pressure-support level, which may represent patient expiratory effort, may be used alone or in combination with the flow threshold for cycling off. There is also typically a time limit set for the duration of inspiration (Brochard and Lellouche 2006).
8.1.3.3.1.3 Physiological Effects (Breathing Pattern, Ventilation/Oxygenation, Work of Breathing)
Because patients can control their own respiratory rate and partially control their own tidal volume, PSV would seem to provide a more “physiologic” means of ventilatory assistance (Brochard and Lellouche 2006). However, the use of PSV actually changes the pattern of spontaneous breathing (Brochard et al. 1989; Ershowsky and Krieger 1987; Tokioka et al. 1989; Van de Graff et al. 1991; Hurst et al. 1989; MacIntyre 1987). Most patients will have an increase in tidal volume with a decrease in respiratory rate as the level of pressure support increases (MacIntyre 1986; Brochard et al. 1989; Ershowsky and Krieger 1987; Tokioka et al. 1989; Van de Graff et al. 1991). These findings suggest that breathing patterns rapidly change when respiratory muscles are faced with a new workload (Tobin et al. 1986) and imply that PSV can be adjusted according to the patient’s breathing pattern response (Brochard and Lellouche 2006). Breathing patterns may change significantly with increasing PSV; however, minute ventilation may increase slightly or not change at all (Brochard et al. 1989; Ershowsky and Krieger 1987; Tokioka et al. 1989; Van de Graff et al. 1991; Hurst et al. 1989).
Gas exchange in PSV can be improved by enhancing alveolar ventilation, which results from an increased dead space to tidal volume ratio (Brochard and Lellouche 2006). PSV corrects PaCO2 and respiratory acidosis for patients with hypercapnic respiratory failure (Brochard et al. 1989), and in healthy non-intubated patients, PSV of 10 cm H2O decreases PaCO2 significantly (Lofaso et al. 1992). Oxygenation is not significantly affected by changes in PSV compared to other modes of ventilation (Brochard and Lellouche 2006).
A major goal of PSV is to provide respiratory assistance while improving a patient’s work of breathing. The decrease in work of breathing is significant and is proportional to the level of PSV (Brochard and Lellouche 2006). In one study, large swings in esophageal pressure generated only small tidal volumes, but when 20 cm H2O of pressure support was added, small changes in esophageal pressure were related to larger tidal volumes (Brochard et al. 1989). These results support that the addition of PSV improves patient effort. The change in the pressure–volume ratio of the work of each breath decreases progressively with increasing levels of pressure support in a lung model (MacIntyre 1986). Providing 5–10 cm H2O of pressure support may reduce the work needed to overcome resistance in the ventilator circuitry and demand valve (Sassoon et al. 1991–1992). Individual patients have an upper limit of pressure support, above which work of breathing is worsened (Brochard and Lellouche 2006). Excessive pressure support can lead to asynchrony, apnea, desaturation, and ineffective efforts (Ershowsky and Krieger 1987).
8.1.3.3.1.4 Clinical Applications, Advantages, and Limitations
There are no exact guidelines for the clinical use of PSV (Brochard and Lellouche 2006). Adding PSV augments the pressure differences between the alveoli and the ventilator circuit, resulting in higher tidal volumes and inspiratory flow rates than spontaneous breathing alone (Brochard and Lellouche 2006). Often, the PSV is adjusted to achieve a tidal volume of 6–8 mL/kg (Brochard and Lellouche 2006). Assessment of accessory muscle activity and respiratory rate may assist in determining the ideal level of PSV (Brochard and Lellouche 2006; Brochard et al. 1989). Targeting a respiratory rate less than 32 inflation per minute (bpm) may be associated with decreased work of breathing (Brochard et al. 1989); however, a lower target rate (<25 bpm) may prolong weaning duration (Brochard and Lellouche 2006).
PSV is commonly used during weaning and assessment of extubation readiness, although evidence remains mixed. A low level of PSV can be used to simulate spontaneous breathing trials (Brochard and Lellouche 2006). PSV can also be used in combination with SIMV as a means for weaning, but there is not much data to support this (Brochard and Lellouche 2006). Using a low level of PSV has been shown to be equivalent or superior to T-piece trials in adults and infants (Esteban et al. 1997; Farias et al. 2001; Matic and Majeric-Kogler 2004).
Noninvasive ventilation (NIV) delivers gas through a face or nasal mask, providing ventilatory support without endotracheal intubation (Yañez et al. 2008). NIV is beneficial as an alternative to endotracheal intubation for adults with neuromuscular disorders, chronic obstructive pulmonary disease, respiratory distress, and cardiogenic pulmonary edema (Brochard and Lellouche 2006; Antonelli et al. 1998; Minuto et al. 2003). PSV is the usual mode of ventilation utilized with NIV. There is less data available for the use of NIV in children, but the initial studies are promising. In a retrospective series of 114 children with varying disease processes requiring NIV with PSV, Essouri et al. found a 77 % success rate in improving respiratory distress and avoiding endotracheal intubation (Essouri et al. 2005). Similarly, in a randomized, prospective study, Yanez et al. demonstrated improved oxygenation and respiratory effort in children with acute hypoxemic respiratory failure (Yañez et al. 2008). They also had a 47 % reduction in the rate of intubation (Yañez et al. 2008).
The advantages of PSV have been briefly mentioned earlier in this chapter. Patients are allowed to breathe in a more “physiologic” way since they control their own respiratory rate and partially control their own inspiratory time and tidal volume (Brochard and Lellouche 2006). This feature may provide improved patient–ventilator synchrony. In a comparison of SIMV and PSV, patients in PSV demonstrated improved subjective comfort, slower respiratory rates, and reduced muscle work (MacIntyre 1986). PSV also avoids disuse atrophy of respiratory muscles that can often result from controlled modes of ventilation (Sassoon et al. 2004). PSV does not have adverse effects on cardiovascular function in patients after cardiac surgery or with respiratory failure (Brochard and Lellouche 2006).
The primary disadvantage of PSV is that tidal volume is not guaranteed. Delivered tidal volume depends, in part, on patient effort, which may continuously change with modifications in neurologic status (increased/decreased sedation), or altered respiratory mechanics (Kornecki and Kavanagh 2007). Oxygen demand and minute ventilation may also change over time, secondary to fever, stress, or pain, but preset pressure support remains constant (Kornecki and Kavanagh 2007). Additionally, if pressure support is high, a patient will decrease their respiratory rate and tidal volume and increase the risk of barotrauma. If pressure support is low, a patient will increase their respiratory rate and reduce their tidal volume, which will increase oxygen consumption and work of breathing (Marraro 2003). If there is inhomogeneous lung pathology, PSV favors ventilation of better aerated areas without affecting collapsed lung areas, potentially worsening ventilation–perfusion mismatch (Marraro 2003). PSV may not be highly recommended for pediatric or neonatal patients because the tidal volume cannot be controlled from breath to breath (Marraro 2003). Hypoventilation may alternate with hyperventilation. In small patients, maintaining appropriate tidal volume is critical for maintenance of alveolar ventilation and avoidance of ventilator-induced lung injury from volutrauma (Marraro 2003).
Volume support ventilation (VSV) is a volume-targeted form of PSV. Breath by breath, VSV adapts the inspiratory pressure support based on changes of the mechanical properties of the lung to ensure that the lowest possible pressure is used to deliver a preset tidal volume (Marraro 2003). As lung pathology improves, the ventilator automatically adjusts the amount of pressure needed to generate the set tidal volume, avoiding the risk of volutrauma associated with high preset pressures (Marraro 2003).
8.1.3.3.1.5 Asynchrony
Patient–ventilator synchrony is an ideal matching of patient demand with ventilator support. Asynchrony is deleterious for patients because it may lead to increased need for sedation, increased work of breathing, respiratory muscle injury, ventilation–perfusion mismatch, dynamic hyperinflation, delayed weaning, increased length of hospital stay, and higher hospital costs (Nilsestuen and Hargett 2005). Asynchrony occurs in all modes of ventilation when a patient has spontaneous respiratory effort. PSV is thought to provide excellent synchrony with patient needs because it can identify both the beginning and end of a breathing effort (Brochard and Lellouche 2006). However, many types of asynchrony are still seen in PSV when the settings are not appropriate for patient demand. Three primary reasons for dyssynchrony in PSV are inappropriate pressure levels, inappropriate flow delivery, and inappropriate cycling-off criterion (Kacmarek and Chipman 2006).
The overall incidence of asynchrony during PSV in neonatal and pediatric patients is not well documented. In a recent animal study performed in young pigs recovering from lung injury, we found that when animals were healthy, the incidence of asynchrony occurred in 8 % of pneumatically triggered inflation (Heulitt 2012). When inflation were triggered via a neural signal from an EMG signal from the diaphragm, asynchrony was still low at 6 % of inflation. However, when the animal’s lung was injured and after undergoing a recruitment procedure, the incidence of asynchrony in the pneumatically triggered inflation increased to 27 % of all inflation, while the neurally triggered inflation remained at 6 %. The level of asynchrony was directly related to increased response time and trigger delay in the pneumatically triggered inflation.
Inspiratory trigger delay is the time from when a patient starts their breath to when the inspiratory valve opens on the ventilator and the ventilator starts supporting the breath. Thus, it relates to the time from the beginning of inspiratory muscle activity to the beginning of mechanical inflation. This delay can lead to patient–ventilator asynchrony. Figure 8.18 is an example of trigger delay. In this example of recordings of flow, pressure ventilator trigger signal (duration of inspiratory valve opening), muscular response measured by EMG activity of the diaphragm, and volume asynchrony are demonstrated when the inspiratory time is twice the mean inspiratory inflation for other inflation during the same time sequence. As illustrated in this example, the second breath has a markedly prolonged inspiratory time as compared to the breath before and after.

Fig. 8.18
Example of prolonged cycle due to trigger delay. Figure illustrates three inflation with waveforms of airway flow, pressure, and volume and ventilator trigger signal and diaphragm EMG tracing. In this example, trigger delay caused the inspiratory time in the second breath to be greater than two times previous and subsequent inflation causing a prolonged cycle
Table 8.6 lists potential factors affecting duration of trigger delay. These causes relate to ventilator characteristics and settings, patient characteristics, and circuit characteristics and interfaces. Ventilator characteristics relate to the type and setting of the trigger. Flow-triggering systems require less patient effort (and thus have less trigger delay) than pressure-triggered systems (Brochard and Lellouche 2006). Triggering effort is 10–30 % of a patient’s total work of breathing, although the clinical implications of this are unclear (Brochard and Lellouche 2006). Inspiratory trigger delay can vary from 40 to 200 ms among different ventilator brands (Richard et al. 2002; Aslanian et al. 1998). If a patient has intrinsic PEEP, he will have to expend even more effort for triggering (Aslanian et al. 1998).
Table 8.6
Factors affecting duration of trigger delay
Ventilator characteristics and setting | Type and setting of trigger |
Site of signal recording | |
Ventilator valve design | |
Level of pressure assistance | |
Ventilator mode | |
Patient characteristics | Presence of dynamic hyperinflation |
Patient respiratory drive during trigger phase | |
Upper airway resistance limited to noninvasive ventilation | |
Circuit characteristics and interfaces | Additional resistance (e.g., endotracheal tube, ventilator circuit, air sensor, HME) |
Presence of air leaks | |
Accumulation of water in ventilator tubing |
Ineffective triggering is when a patient’s inspiratory effort does not trigger a ventilator breath (Thille et al. 2006). This type of asynchrony is directly influenced by the level of PSV and the amount of dynamic hyperinflation (Brochard and Lellouche 2006). It is probably the most common form of asynchrony seen in patients on PSV. Excessive assistance will lead to hyperinflation of the lungs and will depress respiratory drive because of high tidal volumes and prolonged inspiration beyond the end of patient effort (Thille et al. 2006). If a patient is weak and/or the level of support is too high, he will be unable to decrease pressure or reverse expiratory flow enough to trigger the ventilator, and his effort will be wasted (Brochard and Lellouche 2006). Leung et al. found there were very few ineffective efforts below 60 % of assistance, but the number of ineffective efforts increased steadily as assistance increased (Leung et al. 1997). Respiratory cycles before these wasted efforts had higher tidal volumes and lower inspiratory times, both of which will lead to increased levels of hyperinflation (Leung et al. 1997). In a study by Thille, 85 % of asynchrony events were due to ineffective triggering (Thille et al. 2006). High pressure support levels and high tidal volumes were associated factors for ineffective triggering in these patients (Thille et al. 2006). In a second study, reducing the pressure support level completely eliminated ineffective triggering in 2/3 of patients and decreased tidal volume to 6 mL/kg predicted body weight (Thille et al. 2008). There was no increase in respiratory muscle energy expenditure and alveolar ventilation remained unchanged (Thille et al. 2008).
During auto-triggering, the ventilator is falsely triggered by a signal not related to a patient’s inspiratory effort. This asynchrony is a type of trigger asynchrony and can be caused by leaks in the ventilator circuit, motion in the circuit, cardiac oscillations, or excessively sensitive trigger sensitivity settings (Thille et al. 2006; Imanaka et al. 2000). A sudden increase in respiratory rate, a persistently high respiratory rate, or an absence of airway pressure drop at the start of an inspiration are all clues that the ventilator may be auto-triggering (Brochard and Lellouche 2006).
Two or more ventilator inflation delivered with a single patient effort are called multiple cycles or “double triggering” (Thille et al. 2006). Double triggering is also termed “stacked inflation” and is exemplified when the delta time between the ventilator trigger is less than one half the mean inspiratory time for inflation during time studied. Stacked inflation can occur with and/or without expiratory flow between the triggers. Figure 8.19 is an example of stacked inflation. Auto-triggering is one cause of this type of asynchrony as is a short ventilator refractory period (Brochard and Lellouche 2006). Double triggering often occurs when a patient’s respiratory demand is high and the ventilator inspiratory time is too short (Tokioka et al. 2001). Tokioka et al. described multiple cycles during PSV when the cycling-off criteria were high (Tokioka et al. 2001).

Fig. 8.19
Example of trigger asynchrony with stacked inflation. Figure illustrates two inflation with waveforms of airway flow, pressure, and volume and ventilator trigger signal and diaphragm EMG tracing. In this example delta time between the ventilator triggers is one half of the mean inspiratory time for other inflation in the study. Stacked inflation occur with or without expiratory flow between the triggers
The rate of the pressurization of a breath determines the initial upstroke of the pressure–time curve and is primarily dependent on the initial peak flow rate set on the ventilator (Brochard and Lellouche 2006). Flow dyssynchrony is related to the rise time, patient respiratory drive, and ventilator performance (Brochard and Lellouche 2006). Choosing a low rate of pressurization results in a greater length of time to reach goal levels of PSV and a convex initial airway pressure curve (Kacmarek and Chipman 2006). The longer it takes to reach the set pressure level, the greater the work of breathing for patients with both obstructive and restrictive lung disease (Bonmarchand et al. 1999). Figure 8.20 is an example of the convexity of the initial pressurization. In this example the presence of the convexity occurs after the inspiratory valve closes, and diaphragm muscular activity continues despite this closure. It is important to note that excessively fast pressurization, however, will lead to an initial overshoot beyond the goal pressure level, leading to potential early cycle termination related to high pressure and thus asynchrony (Brochard and Lellouche 2006).

Fig. 8.20
Example of trigger asynchrony with effort without response. Figure illustrates two inflation with waveforms of airway flow, pressure, and volume and ventilator trigger signal and diaphragm EMG tracing. In this example there is evidence of concavity of the pressure waveform. It is important to note that the muscular activity exemplified as the diaphragm activity occurs after the inspiratory valve closes
If the cycling-off criterion is reached too early, the ventilator stops insufflation and opens the expiratory valve, while patient inspiratory effort continues (Brochard and Lellouche 2006). This type of asynchrony is referred to as early cycling off. It occurs when the cycling-off criterion is based on flow decay or time, as it is during PSV (Brochard and Lellouche 2006). If the expiratory valve opens while a patient is still actively inspiring, there is an initial drop in airway pressure and flow, followed by an increase, which results in a characteristic contour to the pressure–time curve (Tokioka et al. 2001). This type of asynchrony causes increased patient effort and prolonged neural inspiratory time (Brochard and Lellouche 2006).
Conversely patient characteristics such as dynamic hyperinflation may lead to delayed expiration which is the difference between the end of a patient’s neural inspiratory time and the termination of inspiration by the ventilator (Brochard and Lellouche 2006). In PSV, the cycling-off criterion is typically a percentage of peak inspiratory flow rate, which often differs between patients with obstructive vs restrictive lung disease (Brochard and Lellouche 2006). Tokioka found an increase in tidal volume and decrease in respiratory rate when the cycling-off criterion was decreased from 45 to 1 %, and work of breathing was less with this low cycling-off criterion as well (Tokioka et al. 2001). This finding suggests that low cycling-off parameters should be considered in patients with acute lung injury (Brochard and Lellouche 2006). However, in patients with obstructive lung disease, a higher cycling-off criteria may be better (Tassaux et al. 2003). Low cycling-off criteria may cause insufflation to continue beyond patient neural inspiratory time, resulting in activation of a patient’s expiratory muscles before the end of the ventilator breath, leading to short expiratory times and increased dynamic hyperinflation (Jubran et al. 1995).
For patients on noninvasive ventilation, prolonged inspiration can also occur, typically due to leaks around a mask (Calderini et al. 1999). The cycling-off criterion can never be reached because of the leaks, and the ventilator continues to deliver a breath until the maximum inspiratory time (several seconds) is reached (Brochard and Lellouche 2006). Patients may then fight the ventilator in this situation (Brochard and Lellouche 2006).
Future Perspectives
Pressure support was introduced as mechanical ventilator mode in the 1980s. Since that time different technical advances have been introduced to allow patients to breathe spontaneously during PSV, for example, by the introduction of flow triggering. Currently patients breathing on PSV have a high incidence of asynchrony. Future advances have focused on redirecting the site of triggering from a generated at the patient’s airway to one from an EMG signal from the patient’s diaphragm. This signal theoretically has the advantage of decreasing trigger delay and allowing a closed-loop system to adjust the level of support to the strength of this signal.
Essentials to Remember
PSV is a patient-triggered, pressure-limited, and flow-cycled form of mechanical ventilation used in patients with intact respiratory drive.
The three major phases of PSV (initiation, pressurization, and cycling off) may vary among ventilators, may be altered by patient effort, and/or may be adjusted by the clinician in some circumstances.
A major goal of PSV is to provide respiratory assistance while improving a patient’s work of breathing. The decrease in work of breathing is significant and is proportional to the level of PSV.
8.1.3.3.2 Proportional Assist
Andreas Schulze and Eduardo Bancalari
Learning Objectives: To Understand
The basic concept of proportional assist ventilation and respiratory mechanical unloading
How the clinician can select ventilator settings with these modes that are specific for the individual type and extent of disease-related derangements in pulmonary mechanics (compliance, resistance, functional residual capacity)
That volume-proportional assist (elastic unloading) is indicated in restrictive lung disease (low lung compliance)
That flow-proportional assist (resistive unloading) is indicated in obstructive airway disease (high airway resistance)
That PEEP influences the functional residual capacity during proportional assist as it does with other modalities
Safety features during proportional assist ventilation such as backup ventilation modes and airway pressure limits
8.1.3.3.2.1 General Aspects
Patient-triggered ventilation typically synchronizes one or two events of the ventilator cycle to certain points in the spontaneous respiratory cycle. For example, the ventilator attempts to identify the onset of a spontaneous breath and subsequently delivers an upstroke in ventilator pressure. Other characteristics of the ventilator pressure contour such as peak inflation pressure and others remain preset by the clinician and may or may not match patient needs. The strategy of “proportional assist ventilation” (PAV) is fundamentally different from the conventional perception of a ventilator being a “pump” that is ignited by a trigger event. With PAV, the ventilator pressure steadily follows a continuous input signal that is derived from the infant’s spontaneous breathing effort. This is achieved by an ongoing servo control of the ventilator pressure. The delivered pressure rises during inspiration in a fashion that is proportionate with a patient-derived signal at any point in time. During full-cycle “respiratory mechanical unloading” (RMU), the airway pressure is servo controlled throughout the entire respiratory cycle, not just during inspiration (Schulze and Schaller 1997). For such proportional amplification modes, the ventilator pressure has to track the input signal virtually without a time lag. This implies that near-perfect synchrony between the patient’s spontaneous effort and the ventilator can be achieved (Fig. 8.21). In addition, a relief in work of breathing ensues.
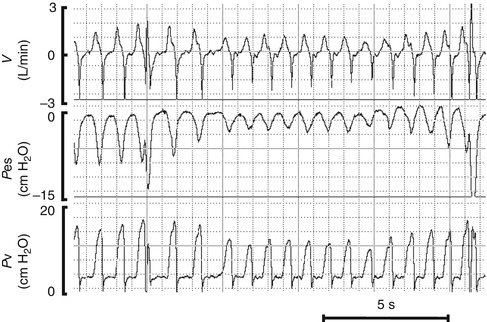

Fig. 8.21
Tracings for a preterm infant supported by proportional assist ventilation. Note the breath-by-breath variability in ventilator pressure which reflects the changing strength of the respiratory effort between inflation in this infant. Sighs (third and last breath on this recording) fail to induce major increases in ventilator pressure because upper ventilator pressure limits are in place as safety feature. The ventilator pressure is aborted to the PEEP level when the upper pressure limit is reached, V’ airflow as measured at the ventilator Y, Pes esophageal pressure, P v ventilator pressure
8.1.3.3.2.2 Clinical Application in Infants
When applying PAV/RMU, the clinician selects three independent settings to address the individual degree of impairment in compliance, resistance, and FRC:
1.
The gain of elastic unloading (volume-proportional assist) which relieves elastic work of breathing for patients with “stiff lungs” (Schulze et al. 1993a). This gain is adjusted on a continuous scale in cm H2O/mL (applied ventilator pressure per unit of tidal volume) (Fig. 8.22).
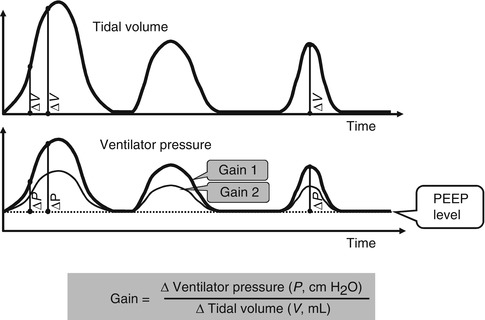

Fig. 8.22
Schematic representation of spontaneous breathing during volume-proportional assist (elastic unloading) at two different settings (gain 1 and gain 2) of the assist gain level. Vertical lines demonstrate that the gain (ratio of change in ventilator pressure per unit of change in tidal volume) is maintained constant over time while the tidal breathing pattern varies. Gain setting two represents a lower level of the assist
2.
The gain of resistive unloading (flow-proportional assist) which relieves resistive work of breathing for patients with obstructive airway disease or those with a high-resistance endotracheal tube (Schulze et al. 1990). This is adjusted in cm H2O/L/s (applied ventilator pressure per unit of airflow) (Fig. 8.23).
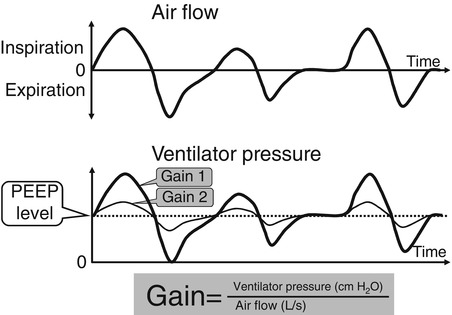

Fig. 8.23
Schematic representation of spontaneous breathing during flow-proportional assist (full-cycle resistive unloading) at two different settings (gain 1 and gain 2) of the assist gain level. Vertical lines indicate that ventilator pressure changes occur virtually without a time lag to the airflow signal. The gain (ratio of change in ventilator pressure per unit of airflow) is maintained constant over time while the tidal breathing pattern varies. Gain 2 represents a lower level of the assist
3.
The PEEP which influences the FRC as it does during conventional mechanical ventilation (Schulze et al. 1993b).
A simple way to initiate PAV/RMU in a clinical setting is to start with zero gains so that the patient briefly breathes without the assist at constant positive airway pressure (CPAP). The elastic unloading gain is then gradually increased to a level judged as “appropriate” by clinical criteria such as reduction in chest wall distortion, physiological tidal volume, and regularity of breathing. It should be considered that smaller infants will need higher gains of elastic unloading (in absolute terms, i.e., in cm H2O/mL) because tidal volume and compliance relate to body weight. As a general rule, infants below 1,000 g of body weight usually need about 1 cm H2O/mL or more of elastic unloading gain while larger infants need less (Schulze and Bancalari 2001). The selected gain of resistive unloading should at least compensate for the resistance imposed by the endotracheal tube. This is about 20–30 cm H2O/L/s of resistive unloading for a 2.5-mm ID endotracheal tube. Higher gains may be required when pulmonary resistance is elevated.
8.1.3.3.2.3 Backup Conventional Ventilation for Apnea and Hypoventilation
An automatic initiation of backup conventional ventilation is required not only during apneic episodes but also in the event of hypoventilation. Small inspiratory efforts will receive little ventilator pressure assist, and hypoventilation will occur if respiratory efforts decrease significantly. PAV modalities for infants should therefore include:
1.
Initiation of backup ventilation in response to cessation of spontaneous breathing. The interval between the last detected breath and the onset of backup should be user adjustable because smaller and sicker infants tolerate less unsupported time.
2.
Initiation of backup in response to a decrease in tidal volume below an adjustable threshold.
3.
Gradual weaning from backup support when spontaneous breathing is resumed. This is necessary because breathing after an apnea is often initially weak and needs time to regain its former strength (Fig. 8.24). The ventilator should provide a feature which allows the user to adjust how fast backup is withdrawn (Herber-Jonat et al. 2006).
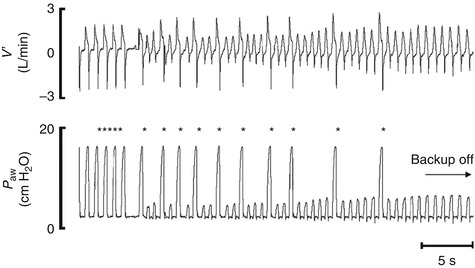

Fig. 8.24
Tracing of airflow (V´), airway pressure (P aw), and oxygen saturation (SpO2) from an 840 g infant during backup ventilation and resumption of spontaneous breathing. Backup is gradually weaned by a stepwise reduction in the mechanical inflation rate until the full spontaneous breathing is recovered. Each spontaneous breath is supported by PAV. Asterisks indicate mechanical backup inflations
8.1.3.3.2.4 Technology
Spontaneous breathing activity drives the ventilator pressure output in the PAV/RMU modes by means of a feedback circuit. The driving signal can theoretically be obtained anywhere along the pathway from the respiratory center to the end organ, i.e., recorded as phrenic nerve activity, diaphragmatic electric activity, but also as tidal volume and airflow signals from probes inside the airway or from plethysmography. In this chapter, proportional amplification techniques will be explained that are based on tidal volume and airflow signals of spontaneous breathing. This is volume-proportional assist ventilation and flow-proportional assist ventilation, which is also called elastic and resistive unloading. Commonly, the driving signals are derived from flow probes mounted between the endotracheal tube and the ventilator Y (Fig. 8.25). Ideally, ventilator pressure changes should occur in phase with the command signal derived from the airflow/volume signals. However, some mechanical delay is dictated by the system’s inherent compliance, resistance, and inertance and will inevitably occur. PAV/RMU for preterm infants with their typical high respiratory rates and very small tidal volumes require exceptionally rapid feedback circuitry, low internal ventilator circuit compliance, and precise airflow/volume tracking.
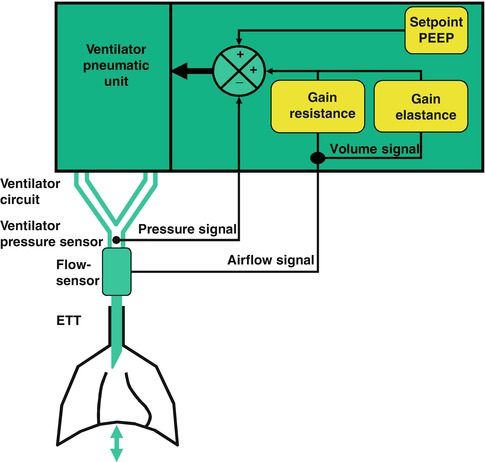

Fig. 8.25
Scheme of a ventilator system for PAV/RMU in infants. Airway pressure is sensed close to the endotracheal tube adapter and the signal is fed into a rapid negative feedback loop. To generate resistive unloading, i.e., flow-proportional assist, the airflow signal as measured at the endotracheal tube adapter is superimposed on the (negative) pressure-feedback circuit such that the airway pressure rises above CPAP during inspiration and decreases below CPAP when there is expiratory flow. To generate elastic unloading, i.e., volume-proportional assist, the tidal volume signal is obtained by integration of the airflow signal. After processing, it is superimposed on the pressure-feedback circuit such that the airway pressure rises in proportion with the tidal volume. The user sets amplification factors (gains, K R in cm H2O/L/s and K E in cm H2O/mL) for resistive and elastic unloading. The generated airway pressure is a servo-controlled, moving variable which is at any point in time a weighted sum of the airflow and tidal volume signal, added to the CPAP set point value 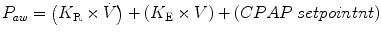 (where
(where 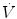 = airflow and V = volume). Because the system is software driven, almost every ventilatory modality can be generated provided that the hardware response times are sufficiently short
= airflow and V = volume). Because the system is software driven, almost every ventilatory modality can be generated provided that the hardware response times are sufficiently short
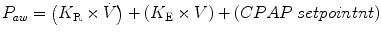 (where
(where  = airflow and V = volume). Because the system is software driven, almost every ventilatory modality can be generated provided that the hardware response times are sufficiently short
= airflow and V = volume). Because the system is software driven, almost every ventilatory modality can be generated provided that the hardware response times are sufficiently shortDepending on the selected gains, the ventilator amplifies the output (which is the ventilation) of the driving signal (which is the respiratory effort) more or less. Similar systems are widely used in electronic engineering. For example, a microphone–loudspeaker system works on the same principle: Sound waves are received, electronically transformed, their signal proportionally amplified, and used to drive the output of a sound wave generator. The gain, i.e., the degree of amplification, can be adjusted to suit individual circumstances.
8.1.3.3.2.5 Terminology and Therapeutic Objectives
While the basic concept of mechanical unloading for conditions characterized by impaired respiratory mechanics dates back to the 1950s (Biernson and Ward 1958), servo-controlled respiratory unloading devices with an appropriate dynamic response for adults (Poon and Huang 1987) and infants (Schaller and Schulze 1991) have been designed since the 1980s.
Investigators have used diverse terminology such as negative impedance ventilation, negative ventilator elastance and negative ventilator resistance, or resistive/elastic unloading (Schulze et al. 1993b). The term “proportional assist ventilation” as introduced in 1992 (Younes 1992) was meant to specifically denote a method of support in which the ventilator provides only inspiratory support in proportion to the instantaneous pressure generated by the respiratory muscles (P mus), i.e., the simultaneous use of both flow- and volume-related assist with gains below the patient’s resistance and elastance.
However, all these devices share a common essential feature: They generate airway pressure in proportion to the instantaneous tidal airflow and/or volume with their respective proportionality factors (weights, gains) selectively adjustable by the user. Nevertheless, the different groups of investigators did have different specific objectives: Younes et al. primarily saw the technique as a means to amplify respiratory muscle effort (P mus) and applied it in adults (Younes et al. 1992). Schulze et al. intended to use an infant ventilator to restore impaired lung mechanics to an “apparently” normal state. They therefore suggested to calculate the magnitudes of “negative ventilator resistance and elastance” required to specifically compensate for the degree of an individual patient’s obstructive and/or restrictive lung disease. This concept of returning apparent respiratory mechanics back to normal also implied the use of unloading throughout the entire respiratory cycle (Schulze et al. 1990, 1993a).
8.1.3.3.2.6 Physiological Effects of PAV/RMU
8.1.3.3.2.6.1 Effect on Elastance
“Elastance” (E) describes a behavior of a system in which a certain change in pressure occurs necessarily with a given change in volume. It can be measured in units of cm H2O per mL. Lung elastance (E l) causes a rise in lung elastic recoil pressure per unit of tidal volume gained. During a normal inspiration, a decreasing pleural pressure needs to be generated by the respiratory muscles to overcome the rising lung elastic recoil pressure. The inspiratory rise in elastic recoil pressure can be opposed by a rise in positive airway pressure. If the rise in positive airway pressure is applied by a ventilator such that it increases in proportion to the tidal volume, the ventilator itself exerts an “elastic behavior” characterized by the change in ventilator pressure per unit of tidal volume. This elastance of the ventilator (E v) is equally quantifiable in cm H2O/mL. However, while lung elastic recoil pressure tends to collapse the lung, the ventilator pressure acts in the opposite direction in the elastic unloading mode. Relative to lung elastance, the elastic “behavior” of the ventilator is therefore “negative.” When a patient is connected to a ventilator in the elastic unloading mode, the overall elastance of the combined system of lung and ventilator is equal to the arithmetic sum of the two elastic elements (because they are interconnected “in series”):
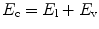 with E v being a negative term.
with E v being a negative term.
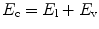
The respiratory muscles work against this overall elastance of the combined system which becomes lower than lung elastance itself when the negative elastic behavior of the ventilator is added. If ventilator elastance is adjusted such that the overall elastance approaches the magnitude of a normal lung’s elastance, respiratory muscles will work on a system of apparently normal elastance. It follows that the patient’s elastic work of breathing will then be in the normal range while the disease-related part of the elastic work of breathing is eliminated by the ventilator. To calculate the magnitude of ventilator elastance needed to achieve a “normal” overall elastance, the equation has to be solved for E v:
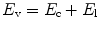
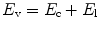
For example, an infant weighs 750 g and has a measured respiratory system compliance of 0.3 mL/cm H2O. The following steps are required to calculate precisely the gain of elastic unloading that returns the infant’s elastic work of breathing to normal:
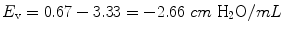
1.
It is assumed that a normal lung of a newborn infant has a compliance of 2 mL/cm H2O/kg. In a 750-g infant, a “normal” lung should therefore have a compliance of 1.5 mL/cm H2O. Because elastance is the inverse of compliance, the target overall system elastance (“normal” lung elastance) should be E c = 1/1.5 = 0.67 cm H2O/mL.
2.
Because the infant’s measured lung compliance was 0.3 mL/cm H2O, the elastance was E l = 1/0.3 = 3.33 cm H2O/mL, the applied ventilator elastance should be
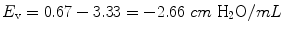
Thus, if the ventilator is set to raise the airway pressure by 2.66 cm H2O for each mL of tidal volume, this infant’s elastic work of breathing would be in the “normal range.”
This example shows that the required level of elastic unloading (gain) in cm H2O/mL depends not only on the absolute stiffness of the lungs but also on body weight. Smaller infants will require higher gains. This is because “normal” lung compliance varies directly with body weight. Because the normal tidal volume in infants also varies directly with body weight, the respiratory muscle pressure amplitude required to generate a normal tidal volume is relatively constant across different body weights.
For clinical purposes, it is sufficient to base the estimate of the required elastic unloading gain on measurements of total respiratory system compliance rather than lung compliance. The former variable is easier to measure because it does not require esophageal pressure manometry. Total respiratory system compliance is not very different from lung compliance in premature infants because of their soft chest wall (high chest wall compliance).
8.1.3.3.2.6.2 Effect on Resistance
Similar to elastic unloading, the applied ventilator pressure waveform during resistive unloading is in close relation to the airflow of spontaneous breathing, and this “behavior” can be described as a “negative resistance,” i.e., a certain change in applied pressure per unit of airflow (cm H2O of ventilator pressure per L/s). For a patient on a ventilator, the overall resistive load on respiratory muscles is the sum of the different components:
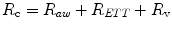 with R aw being the airway resistance, R ETT the endotracheal tube’s resistance, and the ventilator’s resistance R v, the latter being a negative term.
with R aw being the airway resistance, R ETT the endotracheal tube’s resistance, and the ventilator’s resistance R v, the latter being a negative term.
For example, an infant is intubated with a 2.5-mm inner diameter endotracheal tube. This endotracheal tube has an estimated resistance of 20 cm H2O/L/s. If the gain of resistive unloading is set at – 20 cm H2O/L/s, the infant can breathe as if the endotracheal tube’s resistance would not be in place. The ventilator specifically relieves the amount of additional resistive work of breathing associated with this endotracheal tube.
8.1.3.3.2.6.3 Effect on Chest Wall Distortion
Impairment of respiratory mechanics in infants is associated with increased chest wall distortion (CWD). CWD further increases respiratory muscles’ work of breathing because work is “wasted” in distorting the chest. This reduces the efficiency of the respiratory muscles’ to generate ventilation. Compensating for impaired respiratory mechanics by unloading decreases CWD and therefore improves the efficiency of respiratory muscles. This has been demonstrated in small adult and newborn animals who were intubated and had their lungs injured with meconium (Schulze et al. 1998b) and in very low-birth-weight infants with acute respiratory illness (Musante et al. 2001). These studies have also shown that compared to unassisted breathing, a larger fraction of the tidal volume enters the chest compartment while the displacement of the abdominal compartment does not change much with respiratory mechanical unloading. Unloading can therefore provide a “stenting pressure” and stabilize the preterm infant’s highly flexible chest wall during spontaneous breathing.
8.1.3.3.2.6.4 Effects on Control of Breathing
Respiratory center output is increased in lung disease with impaired respiratory mechanics. It has been shown in animal models with severe pulmonary parenchymal failure that PAV/RMU decreases phrenic nerve activity while tidal volume rises and blood gases improve (Schulze et al. 1999b).
8.1.3.3.2.6.5 Overcompensation of Lung Elastic Recoil and Pulmonary Resistance
Magnitudes of unloading that equal or surpass lung elastic recoil (or pulmonary resistance) may induce a runaway of the ventilator pressure. In such situation, a small initial change in volume (airflow) triggers an increase in ventilator pressure higher than the accompanying rise in lung elastic recoil (flow-resistant airway pressure gradient) which in turn further increases lung volume (airflow). This positive feedback self-perpetuates because the negative elastance of the ventilator exceeds the positive elastance of the respiratory system. With such excessive gain settings, artifacts on the volume/airflow signal may initiate ventilator pressure runaway up to the set ventilator pressure limit or a set tidal volume limit. Unless ventilator pressure limits are in place, overcompensation of lung elastic recoil (excessive gains of volume-proportional assist) will lead to a self-perpetuating passive inflation. This process will not be terminated until overinflation leads to stiffening of the lung (increased lung elastance, decreased lung compliance) so that the ventilator pressure finally equals lung elastic recoil pressure (lung elastance equals the magnitude of the set “negative” ventilator elastance). As lung recoil pressure is completely balanced by the ventilator pressure at this point, passive expiration will still not ensue even though the inspiratory effort may be long over. Therefore, besides tidal volume limits, an airway pressure limit and an inflation time limit should always be in place during PAV/RMU (Schulze et al. 1998a).
Full compensation of pulmonary resistance (when negative ventilator resistance equals pulmonary resistance in magnitude) induces sinusoidal oscillations of the respiratory system near its resonant frequency. This phenomenon can be explained by electrical or mechanical analogies of the respiratory system. The amplitude of the oscillations increases with higher gains of resistive unloading so that a variant of high-frequency oscillatory ventilation near the resonant frequency may be generated (Schulze et al. 1991).
8.1.3.3.2.6.6 Effect of Endotracheal Tube Leaks
A leak around the endotracheal tube will be measured as inspiratory airflow and volume by the pneumotachograph that is mounted at the endotracheal tube adapter. In the PAV/RMU modes, this will cause a rise in airway pressure out of proportion to the airflow and volume entering the lung. In case of a substantial leak, the ventilator pressure will rise rapidly to the set pressure limit and then fall to the PEEP level and may rise again repetitively. However, the leak component of the airflow signal can be estimated by the ventilator so that the airway pressure changes can then be based on a corrected flow signal (Fig. 8.26). With appropriate algorithms, PAV/RMU devices for infants can perform satisfactorily in the presence of variable leaks of up to 20–30 % of the tidal volume.
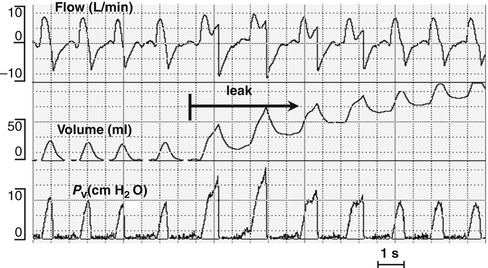

Fig. 8.26
Sudden opening of a substantial endotracheal tube leak during proportional assist ventilation: Note that the ventilator pressure increases during inspiration out of proportion with the tidal volume signal and reaches the upper ventilator pressure limit. Four inflation later, however, it returns to its pre-leak contour although the leak persists. An algorithm in the ventilator software recognizes the air leak, calculates its average size, and subsequently subtracts leak flow from the measured total inspiratory airflow. Recording from a normal piglet
8.1.3.3.2.7 Studies on PAV/RMU in Small Animal Species and Infants
PAV/RMU has been extensively studied in small adult and newborn animals with normal lungs and in various models of neonatal lung diseases such as surfactant deficiency and meconium aspiration. A clinical study on preterm infants compared PAV to assist control and to conventional mechanical ventilation in a crossover design in 36 infants (body weight 600–1,200 g) with mild to moderate acute respiratory illness (Schulze et al. 1999a). Short-term physiological outcome variables were evaluated. During PAV, required mean airway pressures and transpulmonary pressures were lower at equivalent oxygen exposure, equivalent pulmonary oxygen uptake, and a similar CO2 removal rate. Under the strictly controlled conditions of the study, PAV appeared safe and at least as effective as the comparison conventional modes. Infants on PAV typically showed a “fast and shallow” respiratory pattern, with respiratory rates about between 50 and 80/min. Tidal volumes were usually smaller than 5 mL/kg. Because preterm infants with their immature renal function are often slightly acidotic, it had been suspected that infants might hyperventilate when enabled to do so with PAV. This, however, was not corroborated. PAV was subsequently investigated in 22 chronically ventilator-dependent preterm infants (mean birth weight 705 g) in a crossover study design with conventional patient-triggered ventilation (Schulze et al. 2007). Ventilator pressure cost of ventilation was statistically significantly lower during PAV compared to the conventional triggered control mode. Recently, improved adaptive backup ventilatory strategies for episodes of cessation of the respiratory drive and oxygen desaturation during PAV have been shown to be effective in preterm infants (Herber-Jonat et al. 2006). In addition, PAV has been used in studies investigating various aspects of the regulation of spontaneous breathing in preterm infants. However, PAV has not been formally evaluated as the initial and sole ventilatory modality in preterm infants with respiratory failure. Also, its utility in infants with other types of pulmonary or cardiac diseases has not been studied.
8.1.3.3.3 Adaptive Support Ventilation
Jean-Michel Arnal
Educational Aims
Understand the physiological background and the principle of functioning.
Know how to set, adjust, and monitor.
Review the evidences for passive and active breathing patients in adult and pediatric.
8.1.3.3.3.1 Adaptive Support Ventilation
8.1.3.3.3.1.1 Introduction
Closed-loop ventilation modes are designed to adjust automatically some settings based on measurement obtained from the patient. A lot of closed-loop control of ventilation (Saxton and Myers 1953; Frumin 1957; Mitamura et al. 1971; Coles et al. 1973; Schulz et al. 1974; Coon et al. 1978; East et al. 1982; Ohlson et al. 1982; Chapman et al. 1985; Rudowski et al. 1991), mechanical pump (Younes 1992; Dojat et al. 1992; Iotti et al. 1996), oxygenation (Yu et al. 1987; East et al. 1991), or combined systems (Mitamura et al. 1975; East et al. 1986; Strickland and Hasson 1991; Waisel et al. 1995) have been described, but few of them are commercially available. Adaptive support ventilation (ASV) is at the present time the only closed-loop ventilation mode to accommodate both for passive and active breathing patients. Based on continuous measurement of pulmonary mechanics, it is conceptually capable of following the disease process automatically and delivers a breath pattern adapted to the respiratory system mechanics.
Japanese authors were the first to report adjustment of tidal volume (V T) and respiratory rate (RR) to control CO2 and to introduce work of breathing (WOB) as a parameter to select the breath pattern (Mitamura et al. 1971, 1975). Laubscher et al. carried the idea further and made the transition from passive to active ventilation an integral part of their adaptive lung ventilation (ALV) and its commercial implementation of ASV (Laubscher et al. 1994a). Although ASV has been available for more than 10 years, clinical experience reported was mainly in the adult population, and pediatric experience remains scarce.
8.1.3.3.3.1.2 Principles of ASV
The basic principle of ASV stems from the work by Otis et al. (1950) and Mead (1960) demonstrating that, for a given level of alveolar ventilation, there is a particular RR that minimize the WOB. This optimal RR is the best compromise between elastic WOB and resistive WOB (Fig. 8.27) and depends on the minute volume (MV), the expiratory time constant (RCexp), and the dead space (V D). To initiate ASV, the clinician sets the patient’s predicted body weight (PBW) and the percentage of minute ventilation (100 % being equal to 100 mL/kg PBW/min in adult patients). V D is computed by the Radford monogram at 2.2 mL/kg PBW. The ventilator then delivers five test inflation to determine RCexp by analysis of the expiratory flow–volume curve (Brunner 2001; Brunner and Iotti 2002; Laubscher et al. 1994b; Brunner et al. 1995; Lourens et al. 2000). Thereafter, the ventilator compute the target V T and RR, and mechanical ventilation starts using an interbreath negative control of V T and RR to drive the patient’s breath pattern to the target V T and RR (Brunner 2002). In passive patients unable to trigger a breath, the ventilator generates pressure-controlled inflation and automatically adjusts the inspiratory pressure (P insp) and timing to achieve the target V T and RR. In active patients able to trigger a breath, the ventilator switch to pressure support inflation, automatically adjusting P insp to achieve the target V T, and it delivers additional pressure-controlled inflation if the patient’s RR is below the target RR. During the maintenance, RCexp is estimated on a breath-by-breath basis to reassess the target and adjust P insp, I:E ratio, and mandatory RR to maintain the target MV and RR within a frame designed to avoid both rapid shallow breathing and excessive inflation volumes. These safety limits to prevent markedly abnormal ventilator settings are automatically determined or can be manually adjusted. The limits are (min–max) as follows: inspiratory pressure (5 cm H2O above PEEP to 10 cm H2O below set Pmax), V T (4.4 mL/kg PBW to 22 mL/kg PBW), mandatory respiratory rate (5/min to 20/RCexp/min), inspiratory time (0.5–2 s), expiratory time (3 RCexp to 12 s), and inspiratory/expiratory time ratio (1:4–1:1). The rather high value of 22 mL/kg PBW for the upper limit of tidal volume can be set at a lower level to avoid lung overdistension by setting P max.
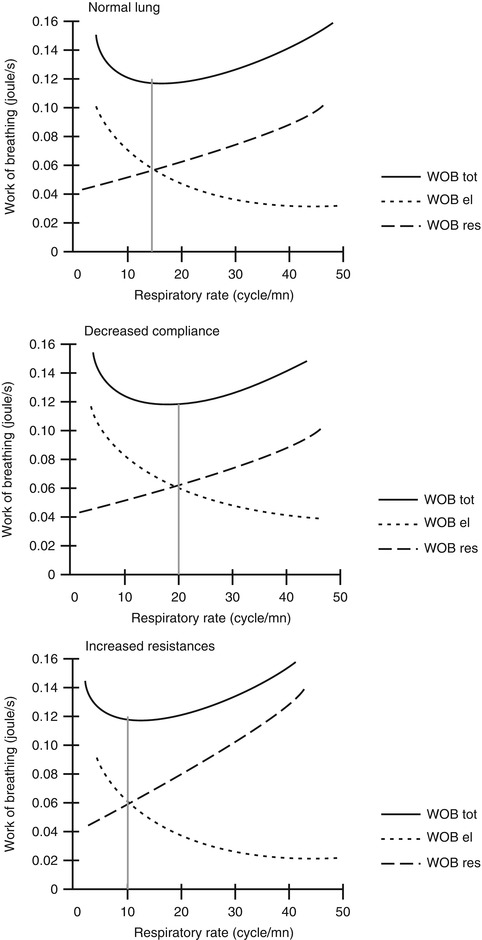

Fig. 8.27
Principle of ASV. ASV is based on Otis’ physiological work (Otis et al. 1950) which determined that for a given minute ventilation, resistive work of breathing (WOB) increases when respiratory rate (RR) increases (dotted line). Reversely, elastic WOB decreases when RR increases (dash line). Thus, total WOB (resistive WOB + elastic WOB) (plain line) has a U shape with an optimal RR associated with the least WOB (grey line). Upper panel is for normal lung patients. Middle panel is the relationship between RR and WOB in decreased compliance patients (e.g., ARDS): the optimal RR is higher than normal lungs. Lower panel is the relationship between RR and WOB in increased resistances patients (e.g., asthma): the optimal RR is lower than normal lungs
8.1.3.3.3.1.3 Settings, Adjustments, and Monitoring
At initiation, clinician has to set MV by setting PBW and %MV. It is crucial to set properly the PBW and not the actual body weight. A large discrepancy between PBW and set body weight may result in large V T delivered (Dongelmans et al. 2008). Usually, %MV is set at 100 % as a starting point (110 % if an HME or heat and moisture exchanger is used on the ventilatory circuit). Later, %MV is adjusted by 10–20 % according to the PaCO2 measured on blood gas analysis. In addition, settings of PEEP, FiO2, P max, inspiratory trigger sensitivity, rise time, and expiratory trigger sensitivity are required. In passive patient, monitoring is similar to the pressure control mode. Plateau pressure is estimated by P insp if inspiratory flow reach zero at the end of inspiration, otherwise an end-inspiratory occlusion is required. Total PEEP is assessed by an end-expiratory occlusion maneuver, especially in asthmatic and COPD patients (Brochard 2002). In active patient, monitoring is similar to pressure support. P insp required to reach the target V T informs on the readiness to wean the patient. If the ASV screen shows that the patient’s RR is far above the target RR, it means a high ventilatory drive that may require increasing the %MV in addition to the etiologic treatment (Wu et al. 2004) (Fig. 8.28). In all cases, monitoring of RCexp inform of the respiratory mechanics. The normal value for intubated adult patient is around 0, 75 s (Arnal et al. 2008). A longer RCexp suggests an obstructive disease (asthma, COPD, bronchospasm, etc.) if not an excess of secretion or an endotracheal tube kicking. A shorter RCexp suggests a restrictive disease either due to the lung (atelectasis, ARDS, etc.) or to the chest wall (morbid obesity, kyphoscoliosis, etc.).
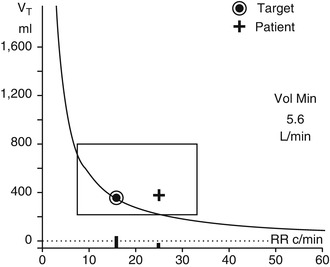

Fig. 8.28
The ASV screen displays respiratory rate (RR) versus tidal volume (V T). In this example, minute ventilation (MV) set is 5.6 L/min. The curve represents all the V T–RR combination to achieve the MV. The circle is the target calculated by the ventilator according to the expiratory time constant. The square is the safety window automatically calculated by the ventilator, which can be also manually adjusted. The cross is the actual breath pattern of the patient. In this case, the patient is actively breathing with a spontaneous RR above the target RR. In order to match spontaneous RR and target RR, clinician can increase the %MV which will increase the ventilatory support
8.1.3.3.3.1.4 Studies in Passive Condition
A multicenter trial tested the safety of ASV as compared to volume control mode (VC) in 48 passive adult patients (Iotti et al. 2005). In term of peak pressure, Pplat, and intrinsic PEEP, ASV was at least as safe as VC. Furthermore, in one-third of these patients, ASV was more effective than VC in clearing CO2. An observational study in a cohort of 243 adult patients with different lung conditions reported different V T–RR combination delivered according to the respiratory mechanics. V T delivered was 8.3, 9.3, and 7.6 mL/kg PBW for normal lung condition, COPD, and ARDS condition, respectively (Arnal et al. (2008). In ARDS patients, a bench study compared V T and Pplat delivered by ASV versus a strict 6 mL/kg PBW strategy (Suleymanci et al. 2008). Result showed that in the most severe cases of low compliance, high PEEP, and high MV, ASV was able to keep Pplat lower than the 6 mL/kg strategy. This is in favor of a good adaptation of the breath pattern delivered according to the respiratory mechanics with a V T delivered below 6 mL/kg PBW for the most severe cases. A subsequent observational study focusing on 51 unselected ARDS patients confirmed the previous result with V T delivered at 7.8 mL/kg PBW on the first day of mechanical ventilation correlated to the static compliance. In addition, Pplat was kept ≤28 cm H2O during all the ventilation duration (Arnal et al. 2006). Two preliminary studies in large numbers of patient report the use of ASV in 98 % of ICU patients (Arnal et al. 2004) with a rate of failure of 3 % (Sviri et al. 2007). To resume, ASV can be used with safety in all conditions of passive ventilated ICU patient. The breath pattern selected is adapted to the respiratory mechanics and is in line with recommendations regarding protective ventilation strategy in ARDS patients (Yilmaz and Gajic 2008).
8.1.3.3.3.1.5 Studies in Active Condition and Weaning
In stable patients spontaneously breathing, the ASV algorithm will progressively and automatically reduce the inspiratory pressure as the patient’s respiratory mechanics improve. A bench study determined that ASV effectively maintains minute ventilation and alveolar ventilation under various conditions of spontaneous ventilation, compliance, and airway resistances (Lawson et al. 2001). A physiological study in ten patients during early weaning with partial ventilatory support suggested an improvement in patient–ventilator interaction and reduced signs of dyssynchrony with ASV compared with SIMV–PS (Tassaux et al. 2002). Another physiological study in 14 adult patient during weaning phase showed that ASV and PSV behaved differently but ended up with similar pressure level facing acute changes in ventilatory demand by contrast to dual modes (volume-guaranteed pressure-control mode), in which an increase in ventilatory demand results in a decrease in the pressure support provided by the ventilator (Jaber et al. 2009).
Preliminary studies in adults suggest that ASV can simplify ventilator management and reduce the time to extubation in predominantly postoperative patient populations. Two RCTs involving 49 and 34 post-cardiac surgery patients, respectively, found that compared to protocols using SIMV and PS, ASV resulted in fewer changes in the ventilator settings from the ICU team providing care (Sulzer et al. 2001; Petter et al. 2003). Additionally, the first one noted that ASV resulted in significantly fewer high-inspiratory pressure alarms (Sulzer et al. 2001), while the second one also noted that ASV significantly reduced the duration of endotracheal intubation [3.2 (2.5–4.6) vs. 4.1 (3.1–8.6) h; P = 0.02] and resulted in more fast-track successes (successful extubation at 6 h) (Petter et al. 2003). Two recent RCT performed in post-cardiac surgery patients found conflicting results (Gruber et al. 2008; Dongelmans et al. 2009). The first one included 50 patients after elective coronary artery bypass grafting and found earlier extubation associated with the use of ASV, without an increase in clinical intervention, when compared with pressure-regulated volume-controlled ventilation (Gruber et al. 2008). The second one compared ASV with pressure support for the post-cardiac surgery weaning in 128 patients and did not find any benefit in terms of ventilation duration (Dongelmans et al. 2009). In all these study, the weaning protocol associated with the use of ASV interfere with the results.
8.1.3.3.3.1.6 Studies in Pediatrics
To test the response of ASV in small pediatric patients with a sizeable endotracheal tube leak, a bench study found that MV delivered was not affected by leaks as high as 50 % for patients whose PBW is 5 kg (Watson et al. 1999). From 10 to 20 kg, MV delivered is affected if leaks are above 25 %. A short-term crossover designed study compared ASV with SIMV in 13 children (mean age 2.3 years, actual body weight 12.3 kg) (Parret et al. 2000). Both V T and peak pressure were decreased with ASV associated with an increase in inspiratory time and a decrease in expiratory time. At the present time, ASV can be used in children in the same way as in adult. In neonates, the experience is too scarce to recommend its use.
Limitations. The absolute contraindication of ASV is an important leak on the circuit as the monitoring of the V T would be disturbed. Thus, ASV is currently not recommended in noninvasive ventilation and in case of bronchopleural fistula. ASV is more and more used around the world both in adult and pediatric population. Still, data are lacking concerning the weaning duration in ICU (beside postoperative patients), workload, cost, and outcomes especially in pediatrics.
Future Perspectives
The rational for using closed-loop ventilation is becoming stronger. It will become unavoidable in ICU aiming to achieve cost-efficiency, quality, and safety. The challenge is how to make that change effortless, friendly, and as fast as possible. Introducing novel graphical user interfaces and providing data displays that are pertinent, integrative, and dynamic will reduce cognitive resources of the clinician and have the potential to make ventilation safer (Wysocki and Brunner 2007). They may be the key for adopting closed-loop ventilation in everyday practice. Furthermore, new closed-loop control will be integrated in the algorithm to control ventilatory pump and oxygenation.
Essentials to Remember
ASV is currently the only closed-loop ventilation mode available for both passive and active breathing patient. The algorithm selects automatically a V T–RR combination associated with the least WOB with designed safety features. Initial settings and adjustments are simple. The monitoring is very similar to conventional ventilation modes. Clinical studies in adult ICU patients have shown a good adaptation of the breath pattern delivered in different lung condition without any safety issue. In some settings, ASV was associated with a faster weaning for post-cardiac surgery patients. Beside many potential advantages, data are still lacking, especially in pediatric population.
8.1.3.3.4 Neurally Adjusted Ventilatory Assist (NAVA)
Guillaume Emeriaud
Educational Aims
To highlight the importance of the electrical activity of diaphragm (EAdi), its physiological relevance, and its recording method
To describe the global concept of neurally adjusted ventilatory assist (NAVA)
To discuss specific points (benefits or problems) concerning NAVA, from the theoretic basics to the clinical data, including patient–ventilator synchronization, concept of diaphragm unloading, and settings of the NAVA level and PEEP
To describe how NAVA could generate clinical benefits, already demonstrated or in future perspectives
To present practical use of NAVA
Mechanical ventilation can induce deleterious effects on lung parenchyma (Dreyfuss and Saumon 1998) or inspiratory muscles (Levine et al. 2008). Emerging evidence suggests that physiological and clinical benefits could be obtained by (1) maintaining spontaneous breathing during mechanical ventilation (Allen 2006; Putensen et al. 2006), (2) avoiding excessive respiratory support (Petrucci and Iacovelli 2007), and (3) reducing patient–ventilator asynchrony (Thille et al. 2006). Pneumatically triggered ventilation modes are universally used, but patient–ventilator synchrony remains poor in both adults and pediatric patients (Thille et al. 2006; Beck et al. 2004). In order to improve patient–ventilator synchrony, Sinderby et al. described in 1999 (1999) the neurally adjusted ventilatory assist (NAVA), a mode that provides a ventilatory support triggered by and proportional to the electrical activity of the diaphragm (EAdi). NAVA has first been developed and extensively evaluated in healthy volunteers and animals. It has recently become available on the Servoi ventilators (Maquet Critical Care, Solna, Sweden) and preliminary experiences of NAVA in ICU patients commence to be reported. In this chapter, the NAVA theoretical concept will first be explained. The potential benefits and problems with NAVA will then be discussed, from theoretic basis to clinical data when available. Last, practical considerations and future perspectives will be presented.
8.1.3.3.4.1 Theoretical Aspects
8.1.3.3.4.1.1 Electrical Activity of Diaphragm (EAdi)
Unlike the conventional ventilatory modes which adapt the assistance to airway pressure or flow, NAVA is adjusted by the neural drive to the diaphragm. The key parameter is the EAdi, which is assumed to reflect the patient respiratory center demand. EAdi signal is continuously recorded using an array of nine miniaturized electrodes mounted on a conventional feeding tube positioned in the lower esophagus (Fig. 8.29). An automated method for online acquisition and processing of EAdi signal has been developed to obtain a high signal-to-noise ratio, to limit electrocardiogram influence, and to maintain an optimal muscle-to-electrode distance despite the diaphragm displacement. Briefly, a cross-correlation analysis of signals measured by each electrode pair permits to identify the diaphragm position (along the array): The most negative correlation value is obtained from the electrode pairs located on either side of the diaphragm (Beck et al. 1996; Sinderby et al. 1997). The signals given by the electrode pairs the closest to the diaphragm are subtracted and filtered, and the remaining heart artifacts are suppressed by replacement (Sinderby et al. 1995, 1998). The final EAdi signal is transferred to the ventilator.
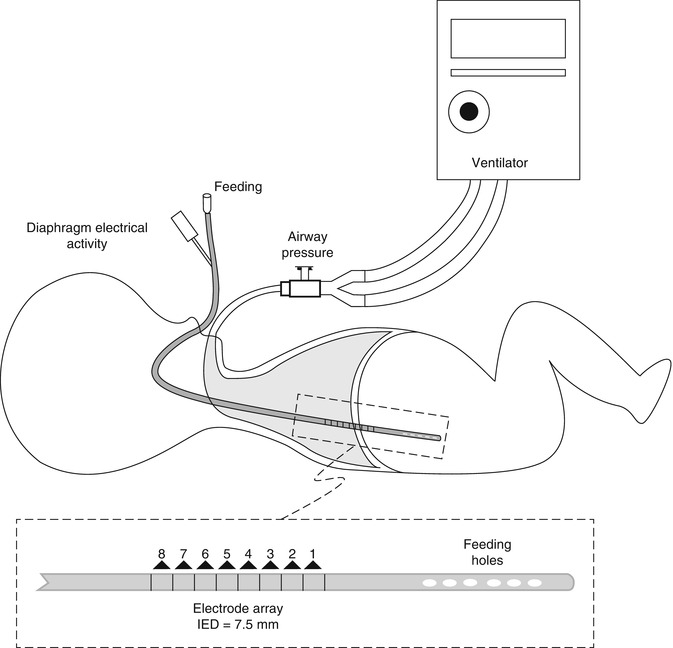

Fig. 8.29
Arrangement of the electrode array on the specific gastric catheter inserted in the lower esophagus
EAdi reflects the respiratory center demand, as illustrated by the EAdi increase observed when the respiratory load is artificially augmented, or in patients with restrictive or obstructive respiratory diseases (Sinderby et al. 1998). Good quality EAdi signals have been successfully recorded in healthy volunteers, critically ill adult patients, pediatric patients including premature infants (Beck et al. 2009; Emeriaud et al. 2006), and small animal species (Allo et al. 2006). Adequate EAdi has also been recorded in patients with severe neuromuscular disease (Beck et al. 2006).
8.1.3.3.4.1.2 NAVA Principle
During NAVA, the ventilatory assist is triggered when EAdi exceeds a threshold level (Fig. 8.30). The magnitude of the mechanical support during inspiration is adapted every 60 ms, corresponding to the EAdi multiplied by a proportionality factor that can be selected, called the NAVA level. Inspiratory support is interrupted when EAdi decreases below 70 % of the peak EAdi, and the selected PEEP is then applied. Experiences of short periods (maximum 8 h) of ventilation with NAVA have been reported in healthy volunteers (Moerer et al. 2008), critically ill adult patients (Brander et al. 2009a; Colombo et al. 2008; Spahija et al. 2010), pediatric patients (Breatnach et al. 2010; Bengtsson and Edberg 2010), premature infants (Beck et al. 2009), and small animal species (Beck et al. 2008; Lecomte et al. 2009).
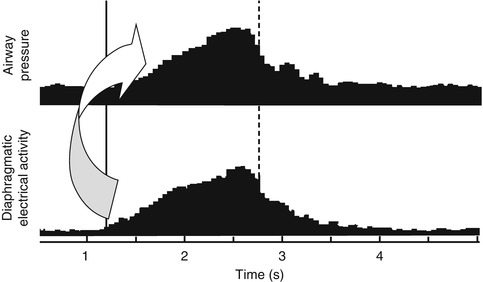

Fig. 8.30
EAdi and airway pressure during a single breath with NAVA. The ventilatory assist is triggered when EAdi exceeds a threshold level (vertical solid line). The magnitude of inspiratory support is proportional to EAdi, with a modifiable gain factor called the NAVA level (arrow). The inspiratory interruption occurs when EAdi decreases below 70 % of peak EAdi (vertical dashed line)
8.1.3.3.4.2 Specific Advantages and Difficulties with NAVA
8.1.3.3.4.2.1 Patient–Ventilator Synchronization
One major advance with NAVA concerns the patient–ventilator synchronization. The electrical activation of the diaphragm precedes its contraction and the modification of pressure or flow in the respiratory circuit; cycling-on delay is intrinsically lower with NAVA than with conventional ventilation based on pneumatic triggering. An important point is that this synchrony should not be affected by the presence of leaks nor by unfavorable mechanical conditions like overdistension with intrinsic PEEP.
The improvement in patient–ventilator synchrony has been confirmed in lung-injured rabbits, with lower trigger delay and fewer non-assisted inflation with NAVA than with pressure-support ventilation (PSV) (Beck et al. 2007). In adult critically ill patients, NAVA was associated with lower asynchrony than PSV, particularly at high level of assistance (Colombo et al. 2008; Spahija et al. 2010); shorter delays were also observed with NAVA for both triggering the assist and cycling off (Spahija et al. 2010). In 7 premature infants (weight 976 ± 249 g), trigger delays were also similar with NAVA or PSV, but the cycling off was more synchronous in NAVA (Beck et al. 2009). Importantly in the same study, the trigger and cycling-off delays in NAVA were not altered after extubation (Beck et al. 2009). In pediatric patients, the synchrony has been less evaluated, but it has been shown that the neural triggering precedes the pneumatic triggering in more than 2/3 inflation during NAVA (Breatnach et al. 2010; Bengtsson and Edberg 2010).
During noninvasive helmet ventilation, an improved patient–ventilator synchrony has also been demonstrated in volunteers (Moerer et al. 2008).
8.1.3.3.4.2.2 Concept of Diaphragm Unloading
The patient–ventilator synchrony is also improved with NAVA in a second dimension: The assist is delivered proportionally in response to the patient respiratory center demand. This relation relies on the concept of unloading the diaphragm during the period of increased respiratory load, in order to prevent a diaphragm fatigue. This concept is quite different than other conventional modes used in pediatric and neonatal patients, like pressure support with volume guarantee, assist-control ventilation, and volume assist. With these modes, when the patient efforts augment, the ventilator progressively decreases its assistance after a few cycles. This can be considered an indication of weaning readiness, but this can also augment the diaphragm fatigue or the oxygen consumption if the situation is prolonged. In contrast, an increase in EAdi during NAVA ventilation is considered like an augmented demand, and the response is a higher assistance. This concept has first been validated in healthy volunteers, with the adjustment of pressure-support amplitude in order to maintain EAdi in an optimal target during exercise (Spahija et al. 2005). Studies in adult (Brander et al. 2009a; Colombo et al. 2008), pediatric (Breatnach et al. 2010; Bengtsson and Edberg 2010), and premature patients (Beck et al. 2009) have confirmed the clinical applicability of NAVA concept. However, the clinical benefit of this concept remains to be demonstrated.
The adjustment of the ventilatory assist to the patient demand implies that this demand is continuously appropriate. Feasibility of NAVA may be impaired in particular during profound sedation. The minimal level of sedation which permits NAVA ventilation has to be studied, but individual evaluation can be done with the monitoring of EAdi during conventional ventilation. A particular concern for pediatric physicians is the immaturity of the central respiratory centers, especially during the neonatal period. Apneas are detected during NAVA, and backup ventilation is activated. However, periodic breathing risks inducing periods of low ventilation. This phenomenon remains to be evaluated, and alarms of minimal ventilation have to be set with caution.
8.1.3.3.4.2.3 Nava Level Setting
The NAVA level is the main ventilator setting during NAVA. This proportionality factor permits to augment the ventilatory support when EAdi increases. There is a theoretic risk of excessive pressure or tidal volume. However, it has been well established in human volunteers (Beck et al. 2001), in animals (Allo et al. 2006; Beck et al. 2008; Lecomte et al. 2009), and in adult patients (Brander et al. 2009b; Colombo et al. 2008; Spahija et al. 2010) that a downregulation prevents excessive ventilation when NAVA level is elevated. When the NAVA level is titrated from zero to high level in cases of acute lung injury (Brander et al. 2009b; Lecomte et al. 2009), a two-phased response is observed (Fig. 8.31). At lower levels, airway pressure and tidal volume progressively increase, unloading the diaphragm as illustrated by a decrease of EAdi which probably return to its normal level. When the NAVA level is further augmented, the EAdi decreases further, illustrating the lower demand, and the inspiratory pressure and tidal volume will plateau. In animals, when NAVA level is at the breakpoint between the two response phases, esophageal pressure, EAdi, and PaCO2 are similar to their baseline normal values.
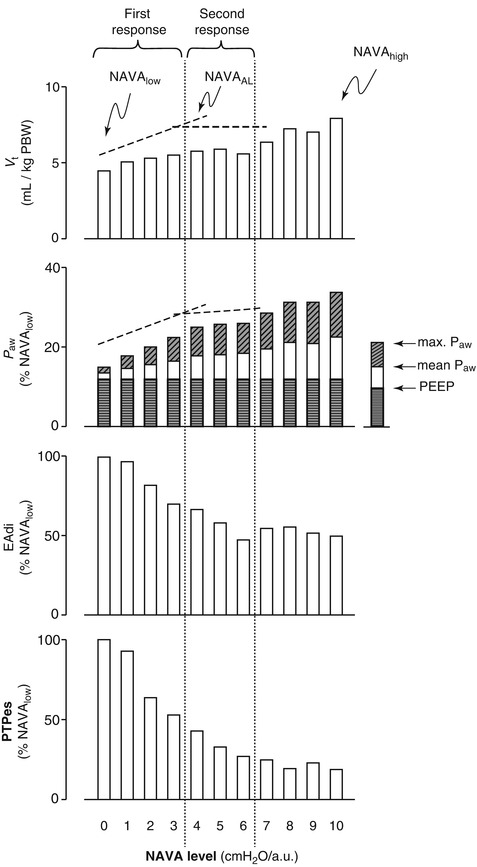

Fig. 8.31
Changes in tidal volume (V t), mean airway pressure (P aw), EAdi, and esophageal pressure–time product (PTPes) during NAVA level titration in a patient. A biphasic response can be observed. At low level of assistance (NAVA low), P aw and V t increase progressively with the NAVA level, with a progressive reduction of EAdi illustrating the diaphragm unloading. Above the breakpoint (considered the adequate NAVA level, NAVAal), V t and P aw reach a plateau despite NAVA level increase, due to the downregulation on EAdi (From Brander et al. 2009a, published with permission)
The ventilatory support adjustment to the patient demand does not result in excessive ventilation. It has been shown, in premature infants (Beck et al. 2009) as in adults (Brander et al. 2009a; Colombo et al. 2008), that patients with acute lung injury ventilated with NAVA spontaneously chose a protective tidal volume (6–7 mL/kg). In pediatric patients, peak inspiratory pressures were lower during NAVA as compared to PSV, with similar minute ventilation (Breatnach et al. 2010; Bengtsson and Edberg 2010). It is however possible that very high NAVA levels induce transiently high tidal volumes or pressures, and corresponding alarms should be activated.
8.1.3.3.4.2.4 PEEP Setting
PEEP setting remains a challenge in intensive care. Two particularities have to be addressed with NAVA. The first point concerns the intrinsic PEEP and hyperinflation. With conventional ventilation, patients have to overcome the intrinsic PEEP before the respiratory effort can be detected. To lower this trigger delay, it is recommended to add an external PEEP. In NAVA, the trigger delay should not be altered by overdistension and this PEEP indication has probably to be reevaluated.
The second point concerns the tonic EAdi. In intubated infants, around 15 % of the inspiratory EAdi remain active at the end of expiration (Emeriaud et al. 2006). This tonic EAdi is probably involved in the control of the end-expiratory lung volume, by braking the expiratory flow. When PEEP is lowered to zero, tonic EAdi increases (Emeriaud et al. 2006; Beck et al. 2008), suggesting that tonic EAdi reflects the patient’s effort to maintain an appropriate end-expiratory lung volume. It can be hypothesized that high levels of tonic EAdi correspond to insufficient levels of PEEP. This remains to be evaluated, but tonic EAdi may become a valuable tool for the adjustment of PEEP in the future.
8.1.3.3.4.2.5 Clinical Benefits
It has to be noticed that no data concerning periods of ventilation superior to eight hours have yet been published. The feasibility of long-term ventilation with NAVA appears good but has to be confirmed, and in particular the stability of EAdi recording.
The improvement in patient–ventilator synchrony is well established with NAVA. The clinical benefits resulting from this synchrony in terms of comfort, sleep quality, sedative use, and ventilation duration have to be evaluated with long-term ventilation periods with NAVA.
NAVA seems also to generate a “safe” ventilation with low tidal volumes. This aspect has recently been confirmed in a study with lung-injured rabbits, in which lower tidal volumes, lower airway pressures, and better PaO2/FiO2 were observed with NAVA as compared to protective conventional ventilation (6 mL/kg), with similar prevention of ventilator-induced lung injury markers (Brander et al. 2009a).
8.1.3.3.4.3 Practical Considerations
NAVA is available on the Servoi ventilators (Maquet Critical Care, Solna, Sweden). All the preparation steps are conducted while the patient breathes on its preceding conventional mode. The first step is the insertion of the special gastric catheter. The correct positioning of the catheter is based on pre-calculated distance of insertion and confirmed using a specific window on the ventilator where the signals of four electrode pairs are displayed. The catheter is correctly positioned when the blue diaphragmatic signals appear mostly in the two middle tracings. Once a correct EAdi signal is displayed in the lower part of the screen, the second step is the setting of the NAVA level. This is generally achieved from a special function which permits to display both actual airway pressure (in conventional mode) and the estimated airway pressure that would result from NAVA ventilation. The NAVA level can be adapted in order to produce the desired pressure. An alternative (but more complex) way to set the NAVA level is to use the titration method described above and in Fig. 8.31. At that point, the NAVA mode can be activated after accurate selection of alarm levels (for maximal pressure, and minimal and maximal minute ventilation).
Of note, the ventilatory assist is triggered by the first activated trigger (EAdi or pneumatic). In case of loss of EAdi signal (e.g., displacement of the tube), pressure-support ventilation will be activated. It is therefore important to correctly set the pressure-support settings. A backup ventilation has also to be set (apneas).
Future Perspectives
NAVA is a new ventilation mode that will probably strongly modify the ventilation practice in intensive care. First experiences with NAVA have only recently been published, and most of the potential benefits are to be studied. Some future perspectives have been mentioned above; the most important one concerns the evaluation of clinical benefits with long-term NAVA. The selection of the patients who will best benefit from NAVA has also to be established. The development of NAVA in noninvasive context seems of paramount importance as well, as this situation is usually associated with a very poor patient–ventilator synchrony in pediatric patients (Essouri et al. 2005). Another perspective relies on the utility of the EAdi signal as a monitoring tool, which reflects very interestingly the patient respiratory center demand.
Essentials to Remember
EAdi is an essential parameter in NAVA, which reflects the patient respiratory center demand. It is continuously recorded with an array of electrodes mounted on a specific gastric tube and with a standardized and automated signal processing algorithm.
During NAVA, the patient–ventilator synchrony is optimized in two dimensions: (1) EAdi is used to trigger (on and off) the ventilatory assist, and (2) the assistance amplitude is proportional to EAdi (with a gain factor, called the NAVA level).
The improved patient–ventilator synchrony with NAVA has been demonstrated in animals, in volunteers, and in adult and pediatric patients. The synchrony is not affected by large leaks – which could be highly valuable for noninvasive ventilation – nor by altered respiratory mechanics like overdistension.
In contrast with other ventilation modes, when the respiratory drive augments in NAVA, the ventilatory assistance is increased. This concept of diaphragm unloading is not associated with excessive ventilation. The tidal volume spontaneously “chosen” by the patients is 6–7 mL/kg in both pediatric and adult patients.
EAdi signal could also be used as a monitoring tool, reflecting the respiratory center demand. The tonic EAdi level may reflect the appropriateness of the PEEP level.
Clinical benefits of long periods of ventilation with NAVA have to be evaluated.
8.1.3.4 Bi-level and Airway Pressure Release Ventilation
Mark J. Heulitt23 and Ronald C. SandersJr.24
(23)
Department of Pediatrics, University of North Carolina, 214 MacNider, CB 7221, Chapel Hill, NC 27599, USA
(24)
Section of Critical Care, Department of Pediatrics, University of Arkansas College of Medicine Arkansas Children’s Hospital, Little Rock, AK, USA
Educational Aims
To understand the physiological principles associated with airway release ventilation/bi-level
To understand the indications and contraindications of its use
To understand how settings are manipulated to effect oxygenation and ventilation
8.1.3.4.1 Introduction
For the purpose of this chapter, we will use the terms airway pressure release ventilation (APRV) and bi-level interchangeable. These modes do differ according to the type of support allowed during the spontaneous inflation, with bi-level incorporating the option of pressure-support in the airway pressure waveform to augment spontaneous breathing. This discussion will be limited to these two modes and not include as reported modes such as BIPAP, IMPRV, and intermittent CPAP. These modes are relatively new, released in the early 1990s (Downs and Stock 1987; Stock et al. 1987), with no studies demonstrating its use reducing mortality or morbidity in ARDS, but has demonstrated improvement in gas exchange at lower airway pressures (Rasanen et al. 1987; Stock et al. 1987). The fundamental principles of both modes differ from conventional ventilation in regard to the starting point of the breath and where within the breath cycle spontaneous breathing can occur. Unlike conventional mechanical ventilation where the breath begins at a baseline pressure and then pressure is elevated to deliver a tidal volume, the APRV breath begins at an elevated baseline pressure and follows with a deflation from high pressure to deliver the tidal volume. Further, and more importantly, spontaneously breathing in contrast to conventional ventilation can occur at either the high-pressure or low-pressure levels. When spontaneously breathing is not present, APRV is not different from conventional time-cycled, pressure-controlled ventilation (Stock et al. 1987; Baun et al. 1989).
8.1.3.4.2 Definition
Practically APRV can be described as the application of continuous positive airway pressure (CPAP) with regular, brief intermittent releases of the airway pressure (Rasanen 1994; Burchardi 1996). However, unlike CPAP, APRV facilitates both oxygenation and CO2 clearance. It is best described using Chatburn criteria (Chatburn and Primiano 2001), as a time-triggered, pressure-limited, time-cycled mode of mechanical ventilation. Theoretically, APRV is capable of complete support in the apneic patient or augmentation of alveolar ventilation in the spontaneously breathing patient. Table 8.7 summarizes the terms utilized in APRV. The degree of ventilatory support with APRV is determined by the duration of the two CPAP levels and the mechanically delivered tidal volume. Even though the terms can be confusing between manufacturers and publications, certain terms and principles are the same. These terms include measures of pressure and time: pressure high (P High) the upper CPAP level analogous to MAP, pressure low (P Low) is the lower pressure setting, time high (T High) is the inspiratory time or phase for the high CPAP level (P High), and time low (T low) is the release time allowing for CO2 elimination (Sydow et al. 1994). P High is the higher of the two airway pressure levels and is the pressure at baseline. The pressure resulting from the pressure release is P Low. T high corresponds with the length of time which P High is maintained; T low is the length of time for which P low is held or the time airway pressure is released (Fig. 8.32).
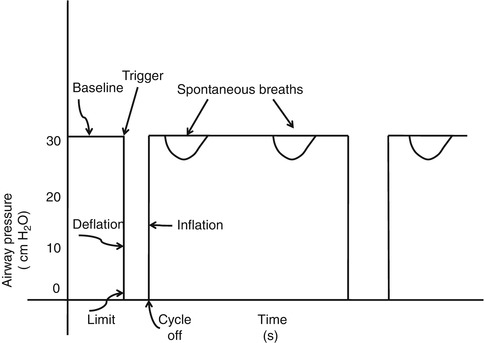
Table 8.7
Airway pressure release ventilation parameters
Term | Definition | Unit of measure |
|---|---|---|
Pressure high (P High) | Baseline airway pressure level Higher of two airway pressures | cm H2O |
Pressure low (P Low) | Airway pressure level resulting from pressure release The lower of the two airway pressures | cm H2O |
Time high (T High) | Length of time for which P High is maintained | Seconds |
Time low (T low) | Length of time for which P low is maintained | Seconds |
Mean P aw | 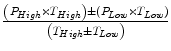 | cm H2O |

Fig. 8.32
Airway pressure release ventilation differs from conventional modes because trigger initiates a drop in airway pressure rather than a rise. The amount of pressure changes will be the limit. The cycle off will occur because of time. Airway pressure then returns to the baseline
8.1.3.4.3 Physiological Effects
Since one of the potential advantages of APRV is to increase the time patients spend breathing spontaneously, we need to review the theoretical advantages of allowing patients to breathe spontaneously during mechanical ventilation. It has been demonstrated that spontaneous breathing provides ventilation to dependent lung regions that get the best blood flow, as opposed to PPV with paralyzed patients (Froese 1974). During spontaneous respiration, there is improved ventilation–perfusion by preferentially ventilating the peri-diaphragmatic regions that receive disproportional blood flow. This effect may vary with the intensity of the breathing efforts and the vigor of the expiratory muscle activity (Kleinman et al. 2002). In patients with ARDS, arterial hypoxemia has been explained by the intrapulmonary shunting due to alveolar collapse in the dependent regions of the lungs demonstrated on computed tomography (CT) (Gattioni et al. 1986). These changes appear to improve with less atelectasis and increased end-expiratory lung volumes as seen in end-expiratory CT of the lungs in experimental ARDS in animals breathing spontaneously in APRV (Wrigge et al. 2003). It has been suggested that the recruitment demonstrated during spontaneous breathing during APRV may be caused by an increase in transpulmonary pressure secondary to a decrease in pleural pressure (Henzler et al. 2004). These effects would lead to reduced atelectasis.
Both animals in experimental models and patients with ARDS breathing spontaneously on APRV have demonstrated improvement in intrapulmonary shunting and arterial oxygenation (Putensen et al. 1999, 1994a, b). Both these settings with improved arterial oxygenation and less atelectasis, thus improved pulmonary compliance, are indicative of lung recruitment. It is important to note that in patients these changes may progress over a period of time and not be instantaneous (Putensen et al. 1999).
Mechanical ventilation with a positive-pressure breath can have detrimental effects on the patient’s cardiovascular status, especially in normo- and hypovolemic patients. This detrimental effect causes decreased stroke volume, cardiac output, and oxygen delivery due to a reduction in right and left ventricular filling. Consequently, any type of ventilatory support that could cause a reduction of intrathoracic pressure would promote venous return to the heart and would reverse these detrimental effects. Reduction in intrathoracic pressure is seen in patients (Putensen et al. 1999; Sydow et al. 1994) and animals breathing spontaneously. Thus, these results would suggest that one should use lower PIPs in order to maintain oxygenation and ventilation without compromising the patient’s hemodynamics (Sydow et al. 1994).
8.1.3.4.4 Clinical Applications
There is little direction on the application of APRV or bi-level. There are theoretical principles within recommendations for protective lung strategy that can be applied to the use of these modes (Artigas et al. 1998; Heulitt et al. 1995; Heulitt and Desmond 1998). First, the clinician must choose initial settings; in these examples we will use examples from Bi-vent, but the principles are the same with other manufacturers. APRV can provide full or partial ventilatory support with low peak airway pressure.
Proper application of APRV requires adjustment of CPAP to a level that results in optimum pulmonary gas exchange and lung mechanics. First select settings for P High, T High, and T PEEP. For P High in adults, this would be the plateau pressure or mean airway pressure in pediatrics. Typically this setting would be about 20–25 cm H2O. In larger patients with P plateau at or above 30 cm H2O, it is recommended to set it at 30 cm H2O. It is important to recognize that overdistension of the lung must be avoided. There is no agreement on what the maximum P High should be, but high settings may be expected in patients that have morbid obesity and decreased thoracic or abdominal compliance (ascites).
For T High the inspiratory time (T high) is set at a minimum of 2.0 s in children and about 4.0 s in older patients. T high is progressively increased (every 10–15 s) with a target being improved oxygenation. These changes should be progressed slowly, for example, 5 s T high to 0.5 s T low, is a 10:1 ratio. So if you increase T High to 5.5 s and T Low to 0.5 s, this only changes the ratio to 11:1. In children use a T High of 2 s. Target is oxygenation.
For release time, it has been proposed in patients with ARDS not to let exhalation go to complete emptying, i.e., do not let expiratory flow return to zero. Thus, regional auto-PEEP is a desirable outcome with Bi-vent. Set T PEEP (T Low) so that expiratory flow from the patient ends at about 50–75 % of peak expiratory flow. This can be determined by saving a screen on the ventilator and calculating the peak expiratory flow from it, or it can be estimated from the ventilator graphics. T PEEP should be set to 0.5–0.8 s in adults and in 0.2–0.6 s in pediatric and neonatal patients. Another way to set it is to use one time constant (TC = C × R). It is important to note that too long a release time could cause the lung to collapse and interfere with the patient’s oxygenation. Thus, it is important to monitor tidal volume and oxygenation so that sufficient time is allowed for reinflation of the lung and that the fall in mean airway pressure and transpulmonary pressure resulting from increasing the release rate does not impair arterial oxygenation. If alveolar ventilation is adequate, but average lung volume is insufficient to maintain oxygenation, the CPAP and the release pressure should be increased simultaneously in equal amounts until the desired effect on oxygenation is seen. In Table 8.8, there are specific recommendations in making ventilatory changes to affect oxygenation or ventilation.
Table 8.8
Strategies used in APRV to change PaO2 and PaCO2
PaO2 | PaCO2 | |
|---|---|---|
Increase | 1. Increase FiO2 | 1. Increase T high (fewer releases/min) |
2. Increase MAP by increasing P High in 2 cm H2O increments | 2. Decrease P High to lower delta pressure | |
3. Increase T high slowly (0.5 s/change) | ||
4. Recruitment maneuvers | ||
5. Maybe shorten T PEEP (T low) to increase PEEPi in 0.1 s. increments (this may reduce V T and affect PaCO2) | ||
Decrease | 1. Decrease T High | |
2. Increase P High to increase delta pressure and volume exchange (2–3 cm H2O/change) | ||
3. Check T low. If possible, increase T low to allow more time for “exhalation” |
8.1.3.4.5 Weaning
FiO2 should be weaned first before any other parameters are weaned. A goal is to reduce FiO2 to 0.50 or less. Weaning the support level of APRV may be accomplished by either reducing the frequency of pressure release or increasing the release pressure so that it is close to the level of CPAP. This is accomplished by manipulation of P High and T High. P High is lowered 2–3 cm H2O at a time to a goal to below 20 cm H2O, and T High will be lengthened in 0.5–2.0 s increments depending upon the patient tolerance to allow for 5 releases per minute. Thus, the patient is transitioned to CPAP with very few releases. It is important to add pressure support judiciously. Pressure support can be added to P High to decrease work of breathing while avoiding overdistension. It is important that P High plus pressure support be maintained less than or equal to 30 cm H2O. Patient needs to be monitored closely to document an increase for the patient’s spontaneous rate to compensate for the APRV weaning.
8.1.3.4.6 Contraindications and Disadvantages
APRV and bi-level can be utilized in a diverse clinical population with acute respiratory failure. Due to the fact that the goal of APRV is to allow the patient to breathe spontaneously during a prolonged phase with increased intrathoracic pressure with a brief release of this pressure, conditions where these physiological effects may adversely affect the patient this mode must be used with caution.
Considerations of the advantages of APRV relates to the limitations of pressure-controlled ventilation, effects of airway and circuit resistance, decrease in transpulmonary pressure, and interference with spontaneous ventilation.
8.1.3.4.7 Pressure-Controlled Ventilation
The generation of tidal volume during APRV is primarily from generation of airway pressure. The magnitude is dependent upon adequacy of inspiratory and expiratory time associated with pressure–volume characteristics of the lungs. Thus, acute changes in pulmonary compliance will alter tidal volume, necessitating clinician-directed changes in settings. This limitation is not unique to APRV, but applies to all forms of pressure-controlled ventilation that has been demonstrated in patients with acute lung injury (Cane et al. 1991).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


