Introduction
Maternal bacterial and viral infections during pregnancy are significant risk factors in several neurobehavioral disorders including cerebral palsy (CP), schizophrenia, and mental retardation. Most recently, epidemiologic studies suggest that common maternal infections, including urinary tract and periodontal, are associated with fetal brain injury and offspring CP. Systemic maternal infections, as evidenced by maternal fever, are also linked to offspring CP. A strong association (odds ratio 9.3) between CP and maternal fever during labor was shown in a study of 46 normal-birthweight children with disabling spastic CP that had not presented with recognized prenatal brain lesions. Recent evidence indicates that subclinical maternal periodontal disease may lead to intrauterine inflammation as well as preterm birth.
Although the mechanisms are largely unknown, our animal studies indicate that simulated maternal infection/inflammation with lipopolysaccharide (LPS) evokes fetal systemic, amniotic, and placental inflammatory responses, increased oxidative stress, and most importantly, increased expression of the proinflammatory cytokine interleukin-6 and the stress-related peptide corticotropin-releasing factor in the fetal brain. Increased cytokine expression in the newborn brain has been associated with long-term brain injury. Using an in vitro culture model, Gao et al demonstrated LPS-activated primary microglia through neuronal nitric oxide (NO) synthase (nNOS) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathways. These results indicate that maternal infection/inflammation alone may induce fetal brain injury through nNOS and NFκB activation.
During the past decade, several prospective studies in pregnant patients suggested a neuroprotective effect of magnesium sulfate (Mg) in preventing white-matter brain injury in preterm birth. Despite human and animal studies suggesting Mg is beneficial, the neuroprotective mechanism of Mg has not yet been elucidated. In accordance with these results and in view of the importance of preventing newborn brain injury we sought to determine whether maternal inflammation will induce activation in fetal brain nNOS and NFκB pathways. We examined proinflammatory cytokines at 2 different gestational ages, embryonic day 16 (E16) and embryonic day 18 (E18), and determined the ability of maternal Mg to attenuate/eliminate this activation.
Materials and Methods
Animals and treatments
Sprague-Dawley pregnant rats (total 48, 6 in each group) (Harlan Sprague Dawley Inc, Indianapolis, IN) were obtained at gestational day 11 (term = 21 days) and allowed to acclimate for 5 and 7 days prior to initiating experiments. Animals were maintained in temperature- (37°C) and light- (0600 lights on; 1800 lights off) controlled facilities with access to food (LabDiet 5001 Rodent Diet; PMI Nutrition International LLC, St. Louis, MO) and water ad libitum throughout the study. Saline (SAL) and Mg were administered subcutaneously and LPS was administered intraperitoneally. LPS ( Escherichia coli serotype 0111:B4; Calbiochem Inc, Darmstadt, Germany) was reconstituted in physiological SAL and administered at 500 μg/kg birthweight.
At gestational days 16 and 18, 48 rats (24 in each gestational age, 6 in each group) were randomized for injections at time 0 of equivalent volumes of intraperitoneal LPS (500 μg/kg) or SAL. Both LPS and SAL were further randomized to treatment with subcutaneous Mg (270 mg/kg loading followed by 27 mg/kg every 20 minutes) or SAL from –2 to +2 hours, followed by an additional injection of Mg (270 mg/kg) at +2 hours. This resulted in 4 study groups: SAL/SAL: SAL-SAL-SAL; LPS/SAL: SAL-LPS-SAL; SAL/MG: MG-NS (normal saline)-MG; and LPS/MG: MG-LPS-MG. To test if Mg facilitates its protective effect through blocking N-methyl-D-aspartate (NMDA)-receptor (R), and to study that mechanism, another group of pregnant rats (n = 5) received 0.3 mg/kg intravenous injections (tail vein) of NMDA-R antagonist (selective for NR1/NR2A) 60 minutes following the LPS injection.
Pregnant rats (6 in each group) were anesthetized 4 hours after the LPS injections, as our previous study demonstrated cytokine peak levels 4 hours following maternal LPS. The hearts and peritoneal cavities were exposed via midline incision. The uterus was removed and placed in a chilled Petri dish. The fetuses in the right lower sac in each dam were decapitated; fetal brains were harvested and immediately frozen in liquid nitrogen for further processing and analyses. We used 1 fetal brain from each dam, resulting in 6 brains from 6 different dams in each group (5 brains in the NMDA-R group). All samples were analyzed individually.
Western blot analysis
Preparations of cell lysates and Western blotting cells or tissues were lysed in RIPA buffer (phosphate-buffered SAL containing 1% Nonidet P-40 [Sigma-Aldrich, St. Louis, MO], 0.1% sodium dodecyl sulfate, 1 mmol/L Na 3 VO1, 4 mmol/L phenylmethylsulfonyl fluoride, and 0.05% [wt/vol] aprotinin). Insoluble proteins were discarded by high-speed centrifugation at 4°C. Protein concentration in the supernatant was measured in triplicate by Nanodrop (Invitrogen, Waltham, MA). Equal amounts of total cellular proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted to nitrocellulose membranes. Antibodies recognizing phospho-nNOS/nitric oxide synthase (NOS) I (Ser 1417), nNOS active form, NFκB, p65 subunit, active subunit (Merck Millipore, Etobicoke, Ontario, Canada) and NMDA-R antagonist selective for NR1/NR2A (Tocris Bioscience, Bristol, United Kingdom) were used in combination with a goat antirabbit and donkey antimouse horseradish peroxidase-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA).
Statistical analysis
Fetal brain phospho-nNOS levels and NFκB p65 units were compared between fetuses from the different groups (6 brains from each group, 1 per dam). All results are expressed as means ± SD using 1-way analysis of variance followed by post hoc tests for pairwise comparisons (Holm-Sidak method). Differences were considered to be significant at P < .05.
Ethical approval
The protocols and procedures were approved by the Institutional Animal Care Committee at the Rappaport Research and Education Institute, Haifa, Israel (protocol number: IL-049-04-2014).
Materials and Methods
Animals and treatments
Sprague-Dawley pregnant rats (total 48, 6 in each group) (Harlan Sprague Dawley Inc, Indianapolis, IN) were obtained at gestational day 11 (term = 21 days) and allowed to acclimate for 5 and 7 days prior to initiating experiments. Animals were maintained in temperature- (37°C) and light- (0600 lights on; 1800 lights off) controlled facilities with access to food (LabDiet 5001 Rodent Diet; PMI Nutrition International LLC, St. Louis, MO) and water ad libitum throughout the study. Saline (SAL) and Mg were administered subcutaneously and LPS was administered intraperitoneally. LPS ( Escherichia coli serotype 0111:B4; Calbiochem Inc, Darmstadt, Germany) was reconstituted in physiological SAL and administered at 500 μg/kg birthweight.
At gestational days 16 and 18, 48 rats (24 in each gestational age, 6 in each group) were randomized for injections at time 0 of equivalent volumes of intraperitoneal LPS (500 μg/kg) or SAL. Both LPS and SAL were further randomized to treatment with subcutaneous Mg (270 mg/kg loading followed by 27 mg/kg every 20 minutes) or SAL from –2 to +2 hours, followed by an additional injection of Mg (270 mg/kg) at +2 hours. This resulted in 4 study groups: SAL/SAL: SAL-SAL-SAL; LPS/SAL: SAL-LPS-SAL; SAL/MG: MG-NS (normal saline)-MG; and LPS/MG: MG-LPS-MG. To test if Mg facilitates its protective effect through blocking N-methyl-D-aspartate (NMDA)-receptor (R), and to study that mechanism, another group of pregnant rats (n = 5) received 0.3 mg/kg intravenous injections (tail vein) of NMDA-R antagonist (selective for NR1/NR2A) 60 minutes following the LPS injection.
Pregnant rats (6 in each group) were anesthetized 4 hours after the LPS injections, as our previous study demonstrated cytokine peak levels 4 hours following maternal LPS. The hearts and peritoneal cavities were exposed via midline incision. The uterus was removed and placed in a chilled Petri dish. The fetuses in the right lower sac in each dam were decapitated; fetal brains were harvested and immediately frozen in liquid nitrogen for further processing and analyses. We used 1 fetal brain from each dam, resulting in 6 brains from 6 different dams in each group (5 brains in the NMDA-R group). All samples were analyzed individually.
Western blot analysis
Preparations of cell lysates and Western blotting cells or tissues were lysed in RIPA buffer (phosphate-buffered SAL containing 1% Nonidet P-40 [Sigma-Aldrich, St. Louis, MO], 0.1% sodium dodecyl sulfate, 1 mmol/L Na 3 VO1, 4 mmol/L phenylmethylsulfonyl fluoride, and 0.05% [wt/vol] aprotinin). Insoluble proteins were discarded by high-speed centrifugation at 4°C. Protein concentration in the supernatant was measured in triplicate by Nanodrop (Invitrogen, Waltham, MA). Equal amounts of total cellular proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted to nitrocellulose membranes. Antibodies recognizing phospho-nNOS/nitric oxide synthase (NOS) I (Ser 1417), nNOS active form, NFκB, p65 subunit, active subunit (Merck Millipore, Etobicoke, Ontario, Canada) and NMDA-R antagonist selective for NR1/NR2A (Tocris Bioscience, Bristol, United Kingdom) were used in combination with a goat antirabbit and donkey antimouse horseradish peroxidase-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA).
Statistical analysis
Fetal brain phospho-nNOS levels and NFκB p65 units were compared between fetuses from the different groups (6 brains from each group, 1 per dam). All results are expressed as means ± SD using 1-way analysis of variance followed by post hoc tests for pairwise comparisons (Holm-Sidak method). Differences were considered to be significant at P < .05.
Ethical approval
The protocols and procedures were approved by the Institutional Animal Care Committee at the Rappaport Research and Education Institute, Haifa, Israel (protocol number: IL-049-04-2014).
Results
Fetuses E16
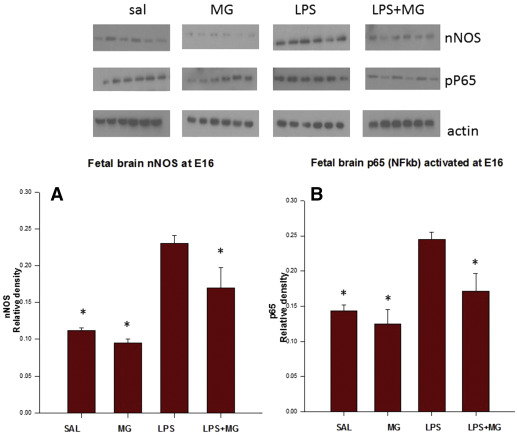
LPS (LPS/SAL) significantly increased fetal brain phospho-nNOS, NFκB p65, and chemokine (C-C motif) ligand 2 (CCL2) protein levels compared to results in the SAL/SAL group at E16 (phospho-nNOS 0.23 ± 0.01 vs 0.11 ± 0.01 U; NFκB 0.24 ± 0.01 vs 0.14 ± 0.01 U; CCL2 0.28 ± 0.01 vs 0.01 ± 0.01 U, P < .05). Maternal Mg to LPS dams (LPS/MG) significantly decreased fetal brain phospho-nNOS, NFκB, and CCL2 protein levels compared to LPS/SAL dams at E16 (nNOS 0.17 ± 0.02 U; NFκB 0.17 ± 0.03 U; CCL2 0.18 ± 0.01 U, P < .05) ( Figures 1 and 2 ).