The rationale for electronic fetal monitoring (EFM) is based on the knowledge that when normal metabolic processes are interrupted, either by a lack of oxygen (O2) or an inability to expel end-products, the subsequent accumulation of acids may damage all or part of the living system.
Fetal well-being depends on adequate functioning of sources and suppliers of oxygen and waste removal mechanisms. These include the maternal system, the placenta, the uterus, and the umbilical cord. At this time, the relationship between specific fetal heart rate (FHR) patterns and fetal acidemia is supported by observational studies only. However, the relationship appears to be strong.
1 It has been well established that a reassuring fetal heart rate tracing is an excellent predictor of the absence of fetal metabolic acidemia.
2 A functional understanding of FHR pattern interpretation inherently and necessarily requires a clear understanding of the relationship between the maternal and fetal chemical and neurologic interactions and exchanges. This chapter explains the physiology of the maternal-fetal unit and relates its functioning to FHR patterns. The specific FHR patterns are discussed in detail in
Chapter 3.
MATERNAL OXYGENATION
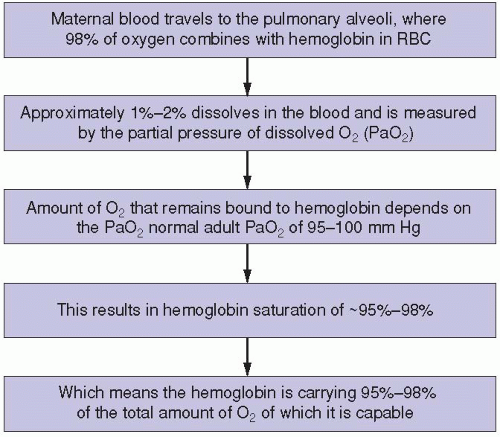
The maternal respiratory system is the only source of oxygen for the fetus. The fetus cannot survive without it (
Figure 1-1). If the maternal oxygen supply or oxygen-carrying capacity is diminished at any level of the process, fetal oxygenation is certain to decrease at some point. This can occur in conjunction with any maternal respiratory, circulatory, hemolytic, or cardiac condition that affects maternal oxygenation. Examples of these include, but are certainly not limited to, asthma, pulmonary embolus, pulmonary edema, pneumonia, hypertension, hypotension, anemia, sickle cell disease, and various forms of cardiac decompensation or insufficiency. To maintain optimal or even sufficient fetal oxygenation, maternal oxygenation must be adequately maintained and supported.
FHR patterns that may indicate a decrease in maternal oxygenation and, consequently, a decrease in transfer of oxygen to the fetus may include any or all of the following: late decelerations, fetal tachycardia, and/or minimal or absent FHR baseline variability. Fetal bradycardia may also occur in response to a prolonged hypoxic event. The physiologic basis of the late deceleration pattern is believed to originate with a decrease in oxygen available to the fetus, most commonly due to a decrease in the amount of oxygen perfused to the fetus through the placenta. The hypoxic fetus may respond to the decrease in oxygen transfer across the placenta that normally occurs during uterine contractions by slowing its heart rate. The FHR then continues at a decreased pace until after the contraction has ended and uterine perfusion returns, re-oxygenating the fetalplacental unit. It is only after blood flow to the fetus has fully resumed (when the uterus has relaxed) that the FHR returns to its baseline rate. The occurrence of this process is demonstrated by the presence of late decelerations in the FHR tracing.
If the fetus becomes hypoxic, rising levels of carbon dioxide (CO2) stimulate the chemoreceptors and increase sympathetic activity, causing the FHR baseline to rise. Due to the effects of these same compensatory mechanisms, loss of variability in the FHR baseline usually accompanies fetal tachycardia.
PLACENTAL CIRCULATION
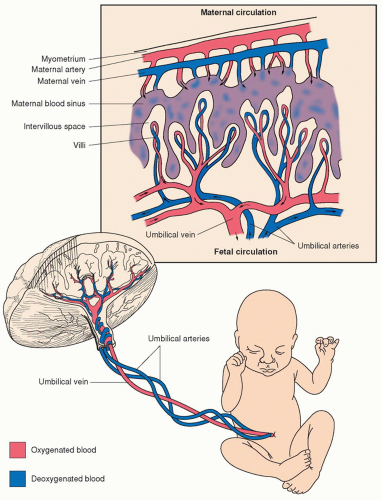
The placenta is the organ that connects the maternal and fetal systems and performs many of the same functions for the fetus that its lungs assume in extrauterine life. The fetus relies on the placenta for transfer of oxygen and nutrients and removal of waste products. The placenta accomplishes this through the villi, which are fetal tissue that project into the maternal blood that is circulating in the intervillous space. It is through these projections that transfer of oxygen, carbon dioxide, and nutrients occurs. Oxygenated blood from the mother is carried to the placenta by the uterine arteries. Blood enters the intervillous space under positive arterial pressure, bathes the fetal villi, and then drains back to the maternal veins (
Figure 1-2).
A microscopic layer of fetal trophoblasts in the placenta serves as a filter, permitting the exchange of nutrients and waste products between the maternal and fetal systems without fetal and maternal blood cells coming into contact with one another. The passage of nutrients and waste products across this membrane occurs due to six mechanisms: facilitated diffusion, passive diffusion, active transport, bulk flow, pinocytosis, and breaks in the system (
Table 1-1). Deoxygenated blood is carried from the fetus to the placenta through the two umbilical arteries. These umbilical arteries split off into smaller capillaries that traverse the fetal villi. The villi project into the intervillous space, where maternal and fetal blood supplies exchange necessary gases (e.g., oxygen and carbon dioxide) and nutrients. Following this transaction, oxygenated blood is carried back into fetal circulation by way of a single umbilical vein.
As the maternal hemoglobin travels to the fetus, there are factors that cause oxygen to be released from the hemoglobin, so that the fetal hemoglobin cells can pick up the oxygen. Some of the factors that cause this release and transfer are:
Increased fetal need for oxygen
Anaerobic glycolysis
Production of hydrogen ions (decreased pH)
Heat
Decreased oxygen transfer from environment to fetus
Severe fetal anemia
Hemoglobinopathies
Due to vasoconstriction, blood flow across the intervillous space is diminished during uterine contractions. This temporary reduction in perfusion forces the fetus to rely on any oxygen that might be available in its system until the contraction ends and normal blood flow resumes. It is similar to the
mechanisms engaged when holding one’s breath to go under water. The vast majority of fetuses show no change in their heart rate or acid-base status during contractions. When placental vasculature and circulation are compromised, however, the fetus is likely to be affected by these episodes of diminished placental blood flow. Examples of maternal health factors that contribute to diminished placental function include, but are not limited to type 1 diabetes, hypertension, and smoking. Other conditions that may compromise the oxygen-carbon dioxide transfer through the placenta include placental dysfunctions, such as those that occur with placenta previa, abruptio placentae, chorioamnionitis, postterm gestation, and intrauterine growth restriction.
Events that may occur transiently, such as uterine tachysystole (six or more contractions in 10 minutes, averaged over a 30-minute window
2,
3) or maternal hypotension, may diminish placental blood flow and lead to the occurrence of late decelerations. Tachysystole has been associated with significant oxygen desaturation.
4 Alleviating transitory causes of uteroplacental insufficiency usually allows the fetus to recover and subsequently remediates the FHR pattern. If the events that are initiating the late deceleration pattern occur repetitively or over a prolonged period of time, oxygen is quickly depleted and the late decelerations become accompanied by other nonreassuring signs, such as tachycardia, decreased variability, bradycardia, and loss of accelerations.
Since maternal and fetal blood supplies are maintained independently, it may be consequential when fetal cells enter the maternal system. A break in the system, such as that which occurs during abruptio placentae, is a common means by which fetal and maternal blood mix. If blood incompatibilities such as Rh, ABO, or other antigen factors are present, isoimmunization can result, and the resulting hemolytic effects on the fetus can be devastating. Since 1976, it has been known that sinusoidal FHR patterns (explained in
Chapter 3) are most often attributable to Rh sensitization or other forms of fetal anemia.
5
UTERINE BLOOD FLOW
The blood flow to and through the uterus is a key determinant in placental function. Approximately 10-15% of maternal cardiac output (˜500-750 mL/min) flows through the uterus of the term gestation. Unlike the rest of the human vascular system, which can constrict and dilate under central nervous system (CNS) control, the uterine vascular bed is believed to constantly maintain maximum dilation. Only an increase in maternal cardiac output can improve uterine blood flow.
Because uterine blood flow is known to decrease during contractions, a diminished amount of oxygen, carbon dioxide, and nutrients is exchanged between fetal and maternal blood during this time. This causes a loss of oxygen to the fetus and a buildup of carbon dioxide within the fetal circulation. Although a healthy, well-oxygenated fetus will have a small reserve of oxygen from which to draw, this will be depleted quickly with repeated episodes of hypoxia. With each uterine contraction and subsequent decrease in perfusion, the fetus without reserve is placed in life-threatening circumstances.
Factors that contribute to decreased uterine blood flow are both iatrogenic and noniatrogenic (
Table 1-2). The noniatrogenic causes include fetal hemorrhage secondary to abruptio placentae, placental deterioration, hypertension, hypotension, autoimmune disease, smoking, tachysystole, and uterine tachysystole. Those that are of iatrogenic etiology commonly occur secondary to treatments and interventions by the perinatal team. For example, administration of oxytocin and other uterotonic agents can lead to excessively long and/or too frequent contractions. Uterine tachysystole may cause late or prolonged decelerations even in the well-oxygenated fetus as a result of the extended period during which uteroplacental blood flow is diminished. In most of these situations, the fetus will have enough oxygen in reserve to recover from the loss of blood flow. A fetus with chronic placental deterioration, however, may not have the ability to recover from an iatrogenic insult such as tachysystole. Tocolytic agents, such as terbutaline, may be used to treat FHR decelerations in the presence of uterine hypertonus or tachysystole. They may also be used in the absence of uterine tachysystole when an abnormal FHR pattern occurs in response to uterine activity. The neonate’s condition may benefit from the improvement in uterine blood flow.
6The administration and management of regional anesthesia can contribute to diminished uteroplacental blood flow. It is of fundamental importance to support maternal circulation and prevent hypotension before, during, and after the administration of medications. Maternal hypotension can cause late decelerations due to the resulting decrease in blood flow to the uterus. The reduction in uterine blood flow potentiates a decrease in blood flow through the uterine arteries, subsequently decreasing blood flow across the placenta. Positioning the patient in the maternal supine position is a common yet avoidable cause of hypotension. The Valsalva maneuver (i.e., maternal breath holding with expulsive efforts during second-stage labor) is another practice to be considered. Various studies have indicated that the resulting maternal hemodynamic effects from breath holding may decrease fetal oxygenation.
7,
8
THE UMBILICAL CORD
Oxygen-rich blood is carried to the fetus from the placenta through the umbilical vein. Deoxygenated blood is carried away from the fetus by two umbilical arteries. Changes in the blood flow through the umbilical cord can impact FHR. Pressure exerted on the umbilical cord can compress the umbilical vein and the umbilical arteries.
9If the umbilical cord is only partially compressed, as occasionally happens during uterine contractions, it is possible for the umbilical vein to be occluded while the arteries remain patent because the walls of the umbilical vein are thinner and more easily compressed than the walls of the arteries. When the umbilical vein is occluded, the flow of oxygen-rich blood to the fetus is diminished. This results in a sympathetic response in the fetus because the change in oxygenation stimulates the chemoreceptors to effect
a transient increase in the FHR. It also causes the fetus to become hypotensive, secondary to hypovolemia, which also stimulates the fetal baroreceptors to trigger an increase in the FHR.
If the umbilical cord becomes further compressed, the umbilical arteries may also become occluded. At this time, as the fetal heart is attempting to pump against a blockade, the FHR slows in order to pump more forcibly. This causes an increase in the fetal blood pressure, and that increased pressure stimulates the fetal baroreceptors. Stimulation of the baroreceptors causes a parasympathetic response; the FHR drops abruptly, resulting in a variable deceleration of the FHR (explained further in
Chapter 3).
As the contraction wanes, cord compression may be progressively alleviated, first relieving pressure on the arteries and leaving only the umbilical vein occluded. The fetal chemoreceptors and baroreceptors again produce a sympathetic response to that change in compression and may transiently accelerate the FHR. These brief accelerations, which can precede, follow, or occur on both sides of a variable deceleration, are an inherent part of the variable deceleration pattern and are referred to as shoulders. This is a benign finding usually associated with moderate FHR baseline variability.
If the fetus is already experiencing compromise, the response to cord compression may be quite different. There may be no acceleration present as the cord is compressed, only the occurrence of a variable deceleration. The variable deceleration is likely to fall far below the FHR baseline in this instance. Additionally, the FHR baseline will likely be tachycardic and have little variability. In an attempt to recover from the variable deceleration to its baseline rate, the compromised fetus first raises its heart rate above the baseline and then effects a very gradual return to baseline rate. These particular types of increases in the FHR that occur as part of the variable deceleration pattern are referred to as
overshoots. When overshoots are present, there is usually minimal or absent FHR baseline variability. Overshoots are considered to be a nonreassuring sign in the term fetus because this pattern is associated with autonomic nervous system (ANS) impairment.
10