Maternal mortality from eclampsia and pre-eclampsia: England and Wales 1952–84; United Kingdom 1985–2005.
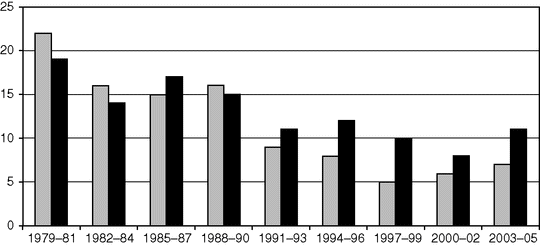
Maternal mortality from eclampsia (grey) and pre-eclampsia (black) in the United Kingdom.
Presentation and Diagnosis
The diagnosis of pre-eclampsia has been greatly improved by vigilant antenatal care, but only around 60% of cases are picked up in this way, with many still presenting acutely with varied signs and symptoms. Although the primary diagnosis is based on hypertension and proteinuria (Table 25.1), these are only signs of the underlying disease, and other wide-ranging complications can be present and virtually any organ system may be affected (Table 25.1). Although these complications can be given other labels such as HELLP (haemolysis, elevated liver enzymes and low platelet count) syndrome, they are all variations of the same underlying disease process and point to severity rather than a different diagnosis [2,20]. These variations contribute greatly to the complications found with this condition, with up to 35% of women having significant morbidities [6].
| 1. Eclampsia | The occurrence of one or more convulsions superimposed on pre-eclampsia |
| 2. Severe pre-eclampsia | Systolic blood pressure over 160 mmHg; or diastolic blood pressure over 110 mmHg (three blood pressure readings in a 15 min period) with at least proteinuria of a + + or >0.3 g in 24 hours. |
| 3. Moderate pre-eclampsia | Systolic blood pressure over 140 mmHg; or diastolic blood pressure over 90 mmHg (three blood pressure readings in a 45 min period) with at least proteinuria + + or >0.3 g in 24 hours. |
| And any of the following | Symptoms of headache; visual disturbance; epigastric pain; signs of clonus; papilloedema; liver tenderness; platelet count falling to below 100 × 109/L; alanine amino transferase (ALT) above 50 IU/l |
Definitions
See Table 25.1. However, it is high blood pressure that is the biggest immediate risk to the mother, and is the most common presenting sign. Even in women who present with other symptoms, such as headache or abdominal pain, it is the elevation of blood pressure that makes the diagnosis and initiates intervention. There is now general agreement that severe hypertension is present if the systolic blood pressure is over 160 mmHg or the diastolic blood pressure is over 110 mmHg on three occasions within a space of 15 min [1,7,21]. Moderate hypertension is present if the systolic blood pressure is between 140 and 159 mmHg and the diastolic blood pressure between 100 and 109 mmHg on three occasions. This is also classified as severe if it is present with significant proteinuria and/or at least two other significant signs or symptoms (Table 25.1). Eclampsia is defined as the occurrence of one or more convulsions superimposed on pre-eclampsia. Up to 40% of women presenting with eclampsia may have no obvious prodromal signs or symptoms, but have a clear diagnosis of pre-eclampsia after the convulsion has occurred [22].
The Problem
Although the classification of pre-eclampsia and its severity is primarily based on the level of blood pressure and the presence of proteinuria, clinicians should be aware of the potential involvement of other organs when assessing maternal risk and the degree of placental disease causing fetal manifestations. It is important that any presenting woman is managed on clinical grounds and not on any perceived presence or absence of a given diagnosis. Many of the maternal deaths and severe morbidity that occur are in women who do not fit the strict criteria of pre-eclampsia. Therefore, clinical signs should not be ignored simply because the precise diagnosis is in doubt.
In the UK, the majority of deaths have been due to cerebral causes, with cardiorespiratory complications being the next most common (Table 25.2). Renal failure, a commonly perceived risk in pre-eclampsia, is a rare event, occurring in around 1/200 cases [6]. It is also a rare cause of death, largely due to the fact that it is not that common, is generally due to acute tubular necrosis which is recoverable, and can be relatively easily managed with dialysis support if required. There has been no maternal death from renal failure in the UK for over 20 years (Table 25.2). This has led to the establishment of guidelines that concentrate on the control of hypertension and the management of fluid balance [7]. Using this form of standardized care package for pre-eclampsia, the Yorkshire series had no maternal deaths in over 1000 cases of severe pre-eclampsia and eclampsia with reduced maternal and neonatal morbidity [6].
| Year | Cerebral | Pulmonary | Hepatic | Renal | Other | Total |
|---|---|---|---|---|---|---|
| 1970–72 | 25 | 8 | 5 | 3 | 6 | 47 |
| 1973–75 | 23 | 7 | 14 | 1 | 4 | 49 |
| 1976–78 | 21 | 4 | 5 | 0 | 3 | 33 |
| 1979–81 | 17 | 8 | 8 | 0 | 3 | 36 |
| 1982–84 | 21 | 3 | 0 | 0 | 1 | 25 |
| 1985–87 | 11 | 11 | 1 | 1 | 2 | 26 |
| 1988–90 | 14 | 10 | 1 | 0 | 2 | 27 |
| 1991–93 | 5 | 11 | 0 | 1 | 3 | 20 |
| 1994–96 | 7 | 8 | 3 | 0 | 2 | 20 |
| 1997–99 | 7 | 2 | 2 | 0 | 5 | 16 |
| 2000–02 | 9 | 1 | 0 | 0 | 4 | 14 |
| 2003–05 | 12 | 0 | 2 | 0 | 4 | 18 |
| 2006–08 | 12 | 0 | 6 | 0 | 2 | 19 |
| 2009–11 | ? | ? | ? | ? | ? | 9 |
| Total | 184 | 73 | 46 | 6 | 41 | 359 |
Although the main disease morbidity for the baby is placental insufficiency and its consequences, the main cause of death is iatrogenic prematurity due to the need to end the pregnancy because of disease severity. The fact that growth restriction is more common in early onset disease means that these problems are additive.
Therefore, the approach on admission should be to assess the severity of the disease and the risk to both the mother and the baby. Blood pressure control should be the first line of management, as it is easy and life-saving. Convulsions, if present, need to be controlled, but the use of prophylactic anticonvulsive therapy is more controversial. Fluid management is more of a problem for intra- and postpartum care, but antenatally, judicious fluid replacement is necessary if delivery is planned. As far as the baby is concerned, emergency delivery on admission is rarely necessary in the absence of placental abruption, but careful assessment of fetal well-being and the likelihood of prolonging the pregnancy needs to be assessed as part of the decision-making process.
How Should Women be Assessed at Initial Presentation?
Many women with severe disease may have few, if any, symptoms and the high blood pressure is discovered as part of routine antenatal care. Others will present with convulsions, abdominal pain or general malaise. As in all medical situations, a clear history and examination should be carried out, and pre-eclampsia should always be considered as a potential diagnosis. The presence or absence of symptoms, particularly headache and abdominal pain, is important, as their presence implies systemic involvement, worsening disease and the increased risk of morbidity. Increasing oedema is a common presenting feature, but is not in itself a sign that should determine management. Enquiry about fetal movement should not be forgotten as an immediate assessment of fetal well-being and as their presence is reassuring.
Examination should start with the diagnostic signs of blood pressure measurement and urine analysis. Abdominal examination should be carried out to assess uterine size and liquor volume, presence of uterine tenderness suggestive of concomitant placental abruption or upper abdominal tenderness suggestive of liver tenderness and HELLP syndrome. Maternal tendon reflexes, although useful to assess magnesium toxicity, are not of value in assessing the risk of convulsion, although the presence of clonus may be. If the woman is extremely unwell, particularly in the postpartum period, she should be assessed for signs of pulmonary oedema, and continuous oxygen saturation monitoring with a pulse oximeter can be invaluable. Auscultation of the fetal heart and commencing of electronic fetal heart monitoring allows further fetal assessment.
An important consideration is the early involvement of senior obstetric and anaesthetic staff and experienced midwives in the assessment and management of women with severe pre-eclampsia and eclampsia. Repeatedly, the Confidential Enquiry into maternal deaths associates the absence of senior involvement with the occurrence of substandard care [4].
How Should the Blood Pressure be Taken?
It is important to take the blood pressure with a cuff of the appropriate size. If in doubt, it is better to use a larger cuff as this will result in less error in a normal size arm than a smaller cuff in overweight women. At least three readings should be carried out and averaged to confirm the diagnosis because of natural variation (see Table 25.1). Korotkoff phase 5 is the appropriate measurement of diastolic blood pressure [23]. Whatever method is used, it should be consistent and documented. Automated methods need to be used with caution, as they systematically underestimate blood pressure readings in pre-eclampsia, especially at higher blood pressure levels [24]. Validation using a mercury sphygmomanometer or other validated device should be carried out if there is concern.
The blood pressure should be checked every 15 min in the acute phase until the woman is stabilized, and then less often depending on the clinical situation.
High blood pressure is the main maternal risk and commencement of antihypertensive therapy if the blood pressure is above 160/100 mmHg is recommended without requiring further assessment. Using the recommended drugs and doses has been shown to be beneficial to the mother and carries no increased risk to the baby [7,25].
How Should the Woman be Monitored?
The presence of proteinuria confirms the diagnosis of pre-eclampsia and systemic involvement, but the level does not differentiate severity and therefore does not need to be repeated once its presence is established [7,26]. Its presence is particularly associated with increased risk to the baby.
The usual screening test for proteinuria is visual dipstick assessment. While it is accepted that there is a poor predictive value from urine dipstick testing [16,27], an approximate equivalence is 1+ = 0.3 g/l, 2+ = 1 g/l and 3+ = 3 g/l. Therefore, generally, a 2+ dipstick measurement can be taken as evidence of proteinuria for clinical management. If a more accurate test is required, this should be a 24-hour urine collection, the ‘gold standard’, or a spot protein creatinine ratio where a level of 0.03 g/mmol appears to be equivalent to 0.3 g/24 hours, the accepted threshold diagnostic level [7].
Therefore, the immediate presumptive diagnosis and presumed severity can be assessed at the bedside: blood pressure and proteinuria measurement along with clinical history and examination. Initial treatment can be instigated without waiting for biochemical, haematological and ultrasound examination.
What Further Tests are Required?
Once a woman has been assessed clinically and any high blood pressure or convulsion managed, a fuller assessment of her and her disease and her baby can be made. This requires a full blood count, liver and renal function tests. These should be repeated as required to assess clinical stability or deterioration. Generally, clotting studies are not required if the platelet count is over 100 × 106/l.
Uric Acid
In pre-eclampsia, there is a rise in uric acid in most cases which helps to confirm the diagnosis of pre-eclampsia and confers an increased risk to the mother and baby, but the absolute levels should not be used for clinical decision making. It is no longer recommended as part of the routine assessment of pre-eclampsia [7].
Urea and Creatinine
Renal function is generally maintained in pre-eclampsia until the late stage. If urea or creatinine is elevated early in the disease process, underlying renal disease should be suspected. There tends to be a rise postpartum, which is of little significance as renal failure is uncommon in pre-eclampsia in the absence of haemorrhage, HELLP syndrome or sepsis.
Platelets
A falling platelet count is associated with worsening disease and is in itself a risk to the mother. However, it is not until the count is less than 100 × 106/l that there may be an associated coagulation abnormality. A count of less than 100 should be a consideration for delivery, as this will tend to fall further, particularly postpartum.
Liver Function Tests
An AST level of above 75 IU/l is seen as significant and a level above 150 IU/l is associated with increased morbidity to the mother. A diagnosis of HELLP syndrome needs confirmation of haemolysis, either by LDH levels or by blood film. These tests are not generally done in the UK, and the diagnosis of HELLP is usually based on platelets and liver function tests (LFTs) alone (ELLP).
Fluid Management
If delivery is planned, close fluid balance with charting of input and output is essential. A catheter with a urometer is helpful during delivery and in the immediate postpartum period. The use of continuous oxygen saturation monitoring with a pulse oximeter can demonstrate early signs of pulmonary oedema [6], the best sign of fluid overload.
How Should the Fetus be Assessed?
After an initial clinical assessment, a cardiotocograph should be carried out. This gives immediate information about fetal well-being and a reactive reassuring tracing suggests that the fetus is not in any immediate danger. Women in labour with severe pre-eclampsia should have continuous electronic fetal monitoring.
The main pathology affecting the fetus is placental insufficiency leading to intrauterine growth restriction (IUGR) in around 30% of pre-eclamptic pregnancies. If conservative management is planned, then further assessment of the fetus using ultrasound should be carried out. Ultrasound assessment of fetal size at presentation is a valuable one-off measurement to assess fetal growth, and can be informative for the decision to deliver, assessment of survival chance and to inform the neonatal unit. Liquor volume should be assessed at the same time, along with umbilical artery Doppler waveform, using absent or reversed-end diastolic flow as a diagnostic criterion [28]. In the presence of normal liquor volume and umbilical Doppler waveforms, continuation of the pregnancy for an average of 15 days is possible if the mother is stable [25]. If abnormalities of fetal assessment are found, prolongation for more than a few days is unlikely, although a course of antenatal steroids should still be attempted if required. Repeat assessment of liquor volume and umbilical artery Doppler waveform can be used along with cardiotocography to assess fetal well-being and optimize delivery. Daily assessment of the changes in umbilical artery and the mid-cerebral artery Doppler waveforms and their ratio can give a more accurate estimate of fetal well-being and decompensation [29].
Pre-delivery Care
General Measures
Initially, the woman should be managed in a high-dependency area, ideally with one-to-one midwifery care. After initial assessment, a decision has to be made about continued management, particularly the need for antihypertensive therapy, magnesium sulphate and antenatal steroids. If delivery is planned, the need for transfer because of fetal and/or maternal care should be considered. This management should be led by the most senior obstetrician present and the consultant should be called to attend. Close liaison with neonatal and anaesthetic colleagues is necessary, again at senior level.
An intravenous cannula should always be inserted, but fluid given with care. A urinary catheter is not always necessary, but may be helpful to monitor urine output over delivery and postpartum.
Antihypertensive Therapy
A blood pressure greater than 160/110 mmHg requires treatment in the maternal interest and many authorities, including the author, recommend that a threshold diastolic blood pressure of over 100 mmHg should be used. Similarly, if the blood pressure is below 160/100 mmHg, there is no immediate need for antihypertensive therapy unless it is associated with other markers of severe disease, such as heavy proteinuria or disordered liver or haematological test results. In these situations, antihypertensive treatment can be used to prevent a hypertensive crisis. Most women can be managed with oral therapy alone [6], with the preferred therapeutic agents being labetalol, nifedipine, hydralazine or methyldopa (Table 25.3) [1,7].
| Labetalol | |
| Oral dose of 200 mg repeated hourly as required. | |
| Daily dose range from 200 mg bd to a maximum of 400 mg qid. | |
| IV bolus dose 50 mg (= 10 ml of labetalol 5 mg/ml) given over at least 1 min. Can be repeated every 10 min if required to a maximum dose of 200 mg. | |
| The pulse rate should be monitored and remain over 60 beats per minute. | |
| IV infusion of neat labetalol at a rate of 4 ml/h via a syringe pump. The infusion rate should be doubled every half-hour to a maximum of 32 ml (160 mg)/h until the blood pressure has reduced and stabilized at an acceptable level. | |
| Nifedipine | |
| Oral dose of 10 mg repeated six-hourly. | |
| After delivery initially this may be changed to the slow-release preparation given 12-hourly. | |
| Hydralazine | |
| IV bolus dose of 5 mg with repeating 5–10 mg IV every 30 min until control is achieved to a maximum of 20 mg. | |
| IV infusion of 0.5–10 mg/h IV to a maximum of 20 mg. | |
| IM dose – intermittent doses of 5–10 mg up to a maximum of 30 mg. | |
| Methyldopa | |
| Oral dose of 250 mg orally usually given three times a day. This can be increased to a 500 mg dose up to four times a day with a maximum daily dose of 2 g. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


