Management of Infections in Patients with Gynecologic Malignancy
AMAR SAFDAR  KENNETH ROLSTON
KENNETH ROLSTON  GENEVIEVE L. BENNETT
GENEVIEVE L. BENNETT
ROBERT PRESS  DONALD ARMSTRONG
DONALD ARMSTRONG
Patients with gynecologic cancer are susceptible to infections. The risk of developing an infection can arise from a number of factors that may relate to the neoplasm and antineoplastic therapy, or both. Tumor encroachment and invasion of adjacent structures, necrosis of expanding tumor mass, surgical tumor excision, and removal of internal organs including lymph nodes and diversion procedures that disrupt protective barriers in the lower and upper female reproductive tract are a few such causes. Patients with these malignancies may also be susceptible to a host of infections due to presence of drug- or radiation-induced mucositis, severe neutropenia, long-term sequelae of prior abdominopelvic radiation therapy, and presence of indwelling foreign devises (1,2).
The wide spectrum of causative microorganisms most frequently arises from endogenous vaginal, urethral, and intestinal tract colonization with bacteria and Candida species. The normal aerobic and anaerobic vaginal bacterial flora may be altered in patients undergoing antimicrobial and antineoplastic therapy. Among factors that influence changes in colonization are hormonal dysfunction, factors influencing alteration in the composition of mucosal secretions including innate immune functions, frequent exposure to broad-spectrum antimicrobial agents, antineoplastic drug and radiation therapy, instrumentation and prolonged, often multiple hospitalizations. In a recent report, however, patients receiving external-beam radiotherapy for gynecologic malignancy did not experience changes in aerobic microflora in the vagina, cervix, or rectum (3,4). Whereas a significant growth in vaginal yeasts occurred during the first 2 weeks after radiation therapy commenced; Candida albicans and Candida tropicalis were prominent. The increase of vaginal growth of Candida species returned to preradiation levels after 4 weeks (5). Exposure to health care facilities, especially centers that care for immunocompromised oncology patients, has been recognized as a major influence, albeit transient in most instances, in promoting change in cutaneous, orointestinal, and genitourinary microflora. The changes in vaginal and lower urinary tract microflora occur less commonly, even in patients following repeated exposure to the hospital environment, and are often limited to patients who undergo instrumentation. The health care–associated microorganisms are frequently less susceptible to commonly prescribed antibiotics, and optimum selection of antimicrobial drugs poses a daunting challenge. It is, however, important to appreciate that changes in a host’s microflora are in most cases transient and restitution of normal intestinal and vaginal flora occurs once factors promoting the change have been removed.
The female genital tract is rich in anaerobic microflora. Peptococcaceae are prominent in the normal vaginal bacterial flora. Quantitative vaginal cultures in recent studies showed that anaerobic bacteria outnumbered aerobic bacteria by nearly 10:1; peptococci, Lactobacillus, Corynebacterium, Eubacterium, and Bacteroides species are frequently isolated (6). Escherichia coli, Klebsiella species, and Enterobacter species are the aerobic Gram negatives, and streptococci, enterococci, and coagulase-negative staphylococcus are gram-positive bacteria isolated from the lower female genital tract. These organisms provide an important reference to local and invasive infections seen in patients undergoing treatment for gynecologic malignancy.
This chapter provides an overview of infections encountered in patients receiving treatment for cancers involving the female reproductive system. A detailed review of specific diseases such as postsurgical abdominopelvic infections, febrile neutropenia, and bacteremia is presented. A focused discussion regarding the changing epidemiology of causative organisms, the increasing rate of infection due to drug-resistant organisms, and an update on new antimicrobial agents is briefly discussed.
PATIENTS WITH FEVER
The factors that increase the risk for infection are shown in Table 29.1. Knowledge of underlying predisposing factor(s) is necessary in the selection of appropriate empiric therapy. Antibiotic prophylaxis and/or recent treatment with broad-spectrum antimicrobial drugs have been associated with the risk of infection(s) due to non-drug-susceptible microorganisms, including systemic candidiasis. Awareness of specific cancer and cancer-therapy–associated risks and the host’s underlying immune dysfunction may allow for proper selection of empiric antibiotic therapy in critically ill patients prior to the results of diagnostic microbiologic and radiographic studies are known. It is important to note that patients with gynecologic cancer may have a high risk of polymicrobial infections due to the presence of physiologic microflora resulting from anatomic proximity to the lower intestinal and urinary tracts. In certain infections, such as deep-tissue abscess, cellulitis in patients with chronic fistula tract, presence of large necrotic tumor, and history of multiple recent instrumentation, the probability of polymicrobial infection remains high (7). Furthermore, in patients with a known anaerobic bloodstream infection, concurrent bacteremia due to aerobic organism(s) is not uncommon (7). Patients with advanced cancer, history of radiation therapy, and extensive pelvic surgery may develop obstruction to the pelvic venous and lymphatic flow. The aberration in physiologic/anatomic defenses and stagnation heightens the risk for cellulitis, abscess formation, and septic thrombophlebitis. Furthermore, these factors also unfavorably influence response to antimicrobial therapy. Several Comprehensive Cancer Centers and other institutions that care for large numbers of patients with cancer have developed Infectious Disease consultative services with a special interest and expertise in infections seen in this high-risk patient population. Table 29.2 provides a breakdown of the various infectious and noninfectious causes that resulted in Infectious Disease consultation requests from the gynecologic oncology clinical service at the MD Anderson Cancer Center.
Predisposing Risk Factors and Infections in Patients with Gynecologic Tumor |
Tumor Related
Obstruction of gastrointestinal or urinary tract
Erosion into bowel, urinary tract, peritoneum, or retroperitoneal space
Necrosis of rapidly growing cancer promotes abscess formation
Lymphatic obstruction
Surgery
Aspiration pneumonia
Hospital-acquired pneumonia, including ventilator-associated pneumonia
Wound infection; skin, and skin structure infection
Tissue necrosis due to disruption of blood supply
Infected hematoma
Fistula tract communication between intestinal and urinary tracts
Complicated peritonitis
Septic deep thrombophelibitis
Fasciitis, myositis, and gas gangrene are rare complications
Chemotherapy
Febrile neutropenia
Pneumonia
Neutropenic enterocolitis
Radiation Therapy
Enteritis
Urinary tract infection
Poor wound healing
Catheter and Implantable Devices
Device infection
Peritonitis
Bloodstream infection
Urinary tract infection
TUMOR RELATED
Tumor-associated infections are dependent on the anatomic site, type, and extent of tumor involvement. In patients with an early-stage, locally involved cancer such as stage I cervical cancer, most infections remain localized to the vagina, whereas in patients with advanced cervical cancer, infections may involve the uterus, fallopian tubes, ovaries and other deep pelvic structures, and retroperitoneum. Infections may present as tubo-ovarian or deep pelvic abscess, endometritis, and less frequently seen pyometra (Figs. 29.1, 29.2, and 29.3). Extension of these infections to adjacent structures may present as ascending pyelonephritis, rectal abscess, and complicated peritonitis, which are often serious requiring surgical debridement or intervention by invasive radiology (8,9). It is also important to note that an infection may be the primary presentation of an undiagnosed malignancy involving the female reproductive tract. In a study of postmenopausal women who presented with tubo-ovarian abscess, nearly half of these patients were subsequently diagnosed with a gynecologic malignancy (10). Therefore, a thorough investigation for possible underlying cancer should be considered in postmenopausal women or those in the absence of sexually transmitted diseases who present with tubo-ovarian abscess or pyometra.
Infectious Diseases Consultations Performed for the Gynecologic Oncology Clinical Service at MD Anderson Cancer Center, Houston, Texas,a n= 373 |
Reason for Consultation | Number (%) |
Intra-abdominal/Pelvic Abscess | 43 (12) |
Surgical Wound Infection | 41 (11) |
Complicated Urinary Tract Infection | 38 (10) |
Neutropenic Fever | 36 (10) |
Pneumonia | 35 (9) |
Unexplained Fever (Including tumor fever) | 35 (9) |
Bacteremia | 32 (9) |
Diarrhea (Including C. difficile associated) | 30 (8) |
Nonspecific Cutaneous Eruption (Rash) | 21 (6) |
Leukocytosis/Leukemoid Reaction | 19 (5) |
Infection with MDR—Organism b | 16 (4) |
Fungemia | 11 (3) |
Varicella-Zoster Infection | 5 (1) |
Meningitis | 2 (1) |
Miscellaneous c | 9 (2) |
a Consultations performed by K.R. from January 2001 to December 2011.
b MDR—Multidrug resistant, defined as resistance to three or more different classes of antimicrobial agents, included cellulitis, and wound breakdown/dehiscence.
c Included travel and other immunizations.
In a large study conducted at the MD Anderson Cancer Center in the 1970s, septicemia, pneumonia, and peritonitis in patients with gynecologic cancer were associated with high infection-associated mortality (11). In the last 2 decades, in patients at an increased risk for infections, early diagnosis, identification of factors associated with poor treatment outcome, empiric therapy with new generation broad-spectrum antimicrobial drugs, advances in antineoplastic and target-centered radiation therapy, and improved surgical techniques have substantially improved outcomes. The recent favorable outcomes for serious systemic bacterial and Candida species infections are in most part due to the availability of well-tolerated broad-spectrum antimicrobial drugs. The recent emergence and spread of multidrug-resistant (MDR) organisms, however, may mitigate these advances, and even patients with solid-organ cancer with limited immune suppression, life-threatening septicemia, pneumonia, peritonitis and abdominopelvic infections may mimic the antibiotic nascent era (11).
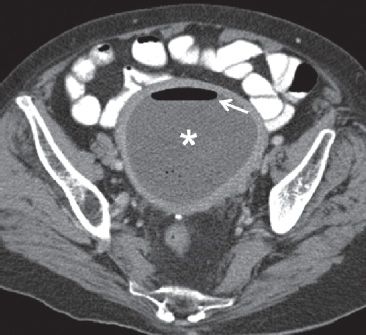
FIGURE 29.1. A 73-year-old woman with endometrial cancer presented with pelvic pain, fever and vaginal bleeding. Axial CT scan performed with enteric and IV contrast demonstrates a markedly distended endometrial cavity containing fluid (*). There is air also present, with an air-fluid level (arrow). In a febrile patient, this is highly suggestive of infection/endometritis. At D&C, blood and pus were drained from the uterine cavity compatible with pyohematometra.
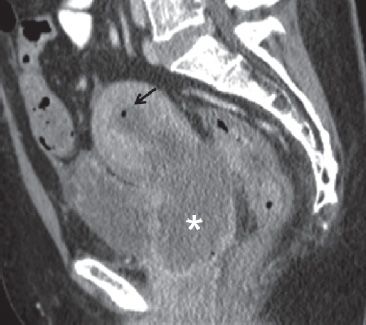
FIGURE 29.2. A 46-year-old woman with cervical cancer presented with pelvic pain and fever. Sagittal contrast enhanced CT image demonstrates a large cervical mass (*). There is obstruction of the endometrial cavity which is mildly distended and contains fluid and air (arrow), compatible with infection/pyometra.
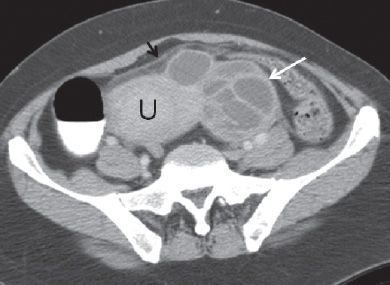
FIGURE 29.3. A 40-year-old woman with cervical cancer presented with fever, left lower quadrant pain and diagnosed with tubo-ovarian abscess. Axial contrast enhanced CT scan demonstrates a complex multiloculated mass with thick septations (white arrow) to the left of the uterine fundus (U). There are inflammatory changes in the pelvic fat (black arrow).
SURGERY RELATED
Patients undergoing surgery for cervical cancer have a higher rate of infectious complications, compared with patients with endometrial cancer who tolerate surgery better and have fewer infections during the early and late postoperative period (12). Pelvic exenteration is performed in patients with advanced and/or treatment refractory cervical and upper reproductive tract malignancies. Infections remain as serious complications following pelvic exenteration; early postsurgical wound infection and wound dehiscence are not uncommon (13,14). Urinary tract infections frequently occur in patients with urinary fistula and/or those who develop urethral obstruction. Studies show that greater than 40% of patients undergoing pelvic exenteration for gynecologic and rectal cancer had urinary tract infection and wound dehiscence, or both (13). Late infections such as ascending pyelonephritis may be serious, as they frequently recur and are usually seen in patients with irreparable structural damage to the urinary reservoir such as those with a neobladder or with stenosis of the urethra or surgical anastomosis site. Ureterointestinal fistula; stones in the urinary reservoir; and surgical, radiation, or chronic/recurring infection/inflammation–induced narrowing all contribute to increased risk of infections, and unless the anatomical abnormality is corrected, these patients remain at increased risk for recurrent urinary tract infections and complications arising from prolonged systemic antibiotic therapy and repeat hospitalizations. Empiric therapy includes adequate coverage for possible polymicrobial infection, and the choice should include drugs that provide adequate coverage for enteric coliforms, enterococci, including vancomycin-resistant Enterococcus species, especially in patients with known prior intestinal or genitourinary tract colonization due to these drug-resistant bacteria and/or receiving care at centers with known high prevalence for these pathogens (15). As most infections in this setting may also have an anaerobe as a copathogen, even in patients with negative microbiologic evidence of anaerobic infection, antibiotic selection should entertain nonisolated anaerobic bacteria. We recommend secondary suppressive antimicrobial therapy in patients with recurrent deep pelvic infection to reduce morbidity and subsequent hospitalization and surgeries. It is critical to select high-risk patients judiciously so patients are not given prolonged courses of broad-spectrum antibiotics, which is the single most important factor in promoting selection for or, less commonly, development of de novo drug-resistant bacteria and fungi.
Infections remain the main concern in patients following radical vulvar resection and inguinal lymph node dissection. In 67 patients undergoing 112 inguinal lymphadenectomy, early postoperative cellulitis is noted in nearly one-third of patients undergoing inguinal lymphadenectomy, and in over 20%, early surgical wound breakdown also occurred (16). Furthermore, they observed late surgery-site cellulitis in patients with chronic lymphedema, whereas, in most patients the surgical wound was not compromised (16).
Wound Infection
Infected surgical wounds in patients following female genital tract surgery include Staphylococcus aureus, including MDR or semisynthetic penicillin resistant S. aureus (MRSA) obtained from health care microflora (health care associated [HA]) or community acquired (CA)-MRSA, which has now become the leading source of MRSA infections in the United States and globally. Streptococcus species (group A, B, C, non-Enterococcus D and G) are also common pathogens in this population, and nearly one-third of infections, especially those with involvement of the lower intestinal tract and urinary tract, are less likely to be monomicrobial.
In certain subpopulations of patients who have been receiving systemic corticosteroids for extended periods, patients with morbid obesity, malnutrition, cancer related cachexia and those with poorly controlled diabetes mellitus have a higher risk of developing postoperative wound infections. Coagulase-negative Staphylococcus, Streptococcus species, S. aureus, enteric gram-negative bacteria, and Bacteroides species are potential pathogens.
Patients who have a complicated hospital course following surgery may develop infections due to organisms acquired from the health care microenvironment. In critically ill patients, infections are treated empirically, and the spectrum of causative organism(s) may not be available at the time of medical decision making. Knowledge of regional and institutional prevalence of drug-resistant organisms is required in prescribing an appropriate treatment regimen. The empiric therapy in patients with prolonged hospitalization and anatomic abnormalities such as enterouretheral, entero- or rectovaginal, or enterovesicular fistula increases the probability of recurrent infection due to resistant HA–gram-negative bacteria, such as Pseudomonas species, and extended-spectrum beta-lactamases–producing and carbapenemases–producing Enterobacteriaceae, such as E. coli and Klebsiella pneumoniae. Other nonfermentative gram-negative bacteria such as nosocomial Acinetobacter spp. and Stenotrophomonas maltophilia are often resistant to a wide variety of commonly used broad-spectrum antibiotics; successful treatment for MDR bacterial disease remains a consternant task (17,18).
Intra-abdominal and Pelvic Abscess
An infected collection within the peritoneal cavity may develop as a consequence of breach in the physical barriers resulting from tumor progression or tumor necrosis and may also occur in patients undergoing instrumentation, or as a consequence of reconstructive surgery and implantation of prosthetic devices. The secondary seeding of the intraperitoneal tumor mass in patients with bloodstream infection arising from a different primary source, such as the urinary tract, antineoplastic therapy–induced orointestinal mucositis, or indwelling vascular device may also occur. The spectrum of causative organisms includes enteric bacteria such as E. coli, Enterococcus, Staphylococcus species, Bacteroides, and other anaerobes. Candida species infection is less frequent in nonneutropenic patients, although patients with high yeast counts in multiple body sites referred as high Candida colonization index, exposure to prolonged broad-spectrum antibiotics, poorly controlled diabetes mellitus, systemic corticosteroid use, as well as stay in critical care units and presence of foreign devices may increase the risk for tissue invasive candidiasis.
Hematogenous or direct seeding of the retroperitoneal space may lead to paraspinal and psoas abscess; these infections escape early detection as patients may not have high-grade fever, and increase in chronic low back pain remains subject to interpretation. In the absence of a direct extension from the intestinal or urinary tract, these retroperitoneal infections are usually monomicrobial, and diagnosis requires prompt computed tomography or magnetic resonance imaging scans and aspiration of the infected collection (Figs. 29.4 and 29.5). In tuberculosis endemic regions, Mycobacterium tuberculosis must be considered in determining the etiology of these infections, especially in patients in whom the vertebral column is involved.
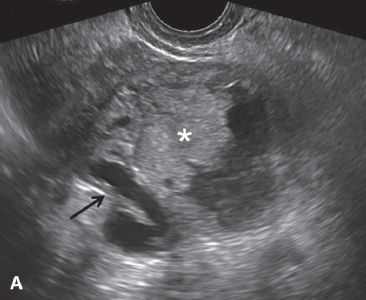
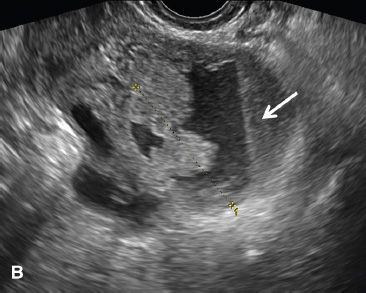
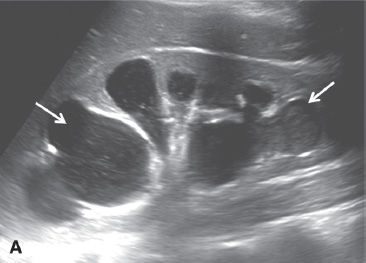
FIGURE 29.4. A 52-year-old woman with fallopian tube carcinoma initially diagnosed as tubo-ovarian abscess. She presented to the emergency department with left lower quadrant pain, and fever. A: Transvaginal ultrasound demonstrates a complex left adnexal mass with a tubular in configuration fluid-filled component (arrow) and a solid component (*).
B: Additional image demonstrates a fluid-debris level within this mass (arrow). Initially, this was thought to represent a tubo-ovarian abscess; however, the patient had no risk factors for pelvic inflammatory disease. At surgery, fallopian tube carcinoma was found.
Pelvic abscesses that develop after instrumentation or following surgery are often polymicrobial, and treatment is directed toward normal intestinal tract and cutaneous microflora.
Peritoneal infections following bowel perforation, anastomotic leaks, or fistula tracts may present with fever, abdominal pain, and fistula drainage and/or surgical wound dehiscence. Most infections, as expected, are polymicrobial, and Enterobacteriaceae, S. aureus, Streptococcus, and Enterococcus species including vancomycin-resistant strains are frequently encountered. Bacteroides fragilis and Clostridium species are common anaerobes; Fusobacterium, Peptostreptococcus, and Eubacterium occur less commonly. Patients with peritoneal infections following surgery involving the nonsterile bowel and lower genitourinary tract may also have an increased risk for Pseudomonas species infection involving the deep pelvic and lower abdominal tissue and/or organs.
CHEMOTHERAPY AND RADIATION RELATED
Patients receiving chemotherapy may have an increased risk of infection; neutropenia for less than a week increases risk for systemic bacterial infections due to S. aureus, Pseudomonas, E. coli and other coliform bacteria. Patients who remain neutropenic for longer than 5 to 7 days are also at increased risk for developing tissue-invasive Candida species infection (1,2). Disruption of orointestinal and genitourinary tract mucosa compromises an important barrier in the prevention of bacterial and fungal invasion. In patients with severe treatment-induced mucositis, α-hemolytic streptococcal and anaerobic septicemia may lead to devastating consequences, including rapidly progressive multiorgan failure, disseminated intravascular coagulation, hemodynamic collapse, and death (19).
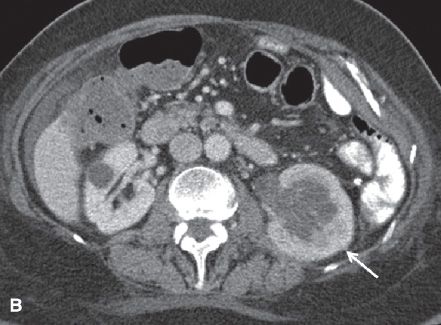
FIGURE 29.5. A 48-year-old woman with cervical cancer presents with left flank pain and fever. A: Sagittal gray-scale ultrasound image of the left kidney demonstrates marked hydronephrosis compatible with ureteral obstruction secondary to the patient’s cervical neoplasm. There is echogenic debris present in the dilated collecting system (arrows) compatible with pus in the setting of pyonephrosis. B: Axial contrast-enhanced CT scan in the same patient demonstrates the enlarged left kidney with hydronephrosis (arrow). There is a delayed nephrogram compared with the right, compatible with obstruction. The left nephrogram is also heterogeneous and there is perinephric inflammatory change compatible with pyelonephritis. The right kidney is normal with the exception of a small cyst.
Radiation therapy in patients with vulvar, vaginal, and cervical cancer causes microvascular damage to the tissue and may lead to difficult-to-treat intestinal, vaginal, and urologic complications.
Increased doses of radiation for gynecologic cancer have been associated with improved cancer-free survival (20). However, with increased radiation dose, the rate of complications has also risen (21). Noninfectious complications arising from high-dose radiotherapy include damage to the organs in radiation field, including necrosis of tissue and bone, fistula formation, enteritis, proctitis, aseptic degenerative arthritis of pelvic, and sacroiliac joints increasing the risk for pathologic fracture. Radiation-induced fibrosis may result in vaginal, rectal, and ureteric stenosis, and predisposes to secondary infection due to stagnation and inadequate physiologic drainage (22). In rare cases, patients with radiation-induced necrosis of pelvic bones may present with osteomyelitis; for these patients, a challenging and multifaceted treatment approach is needed for successful outcomes (23) (Fig. 29.6). A patient with osteomyelitis in the setting of radiation-induced bone necrosis may need extensive surgical debridement and an appropriate selection of antibiotics for a prolonged period. The excisional defects create additional complexity due to placement of prosthetic of autologous or cadaveric biologic grafts. A high clinical suspicion remains critical in the timely diagnosis of radiation-related infectious and noninfectious complications.
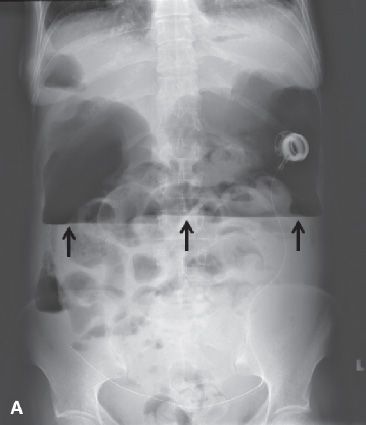
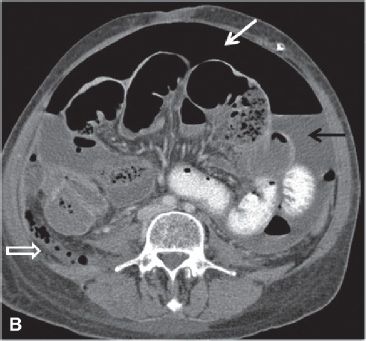
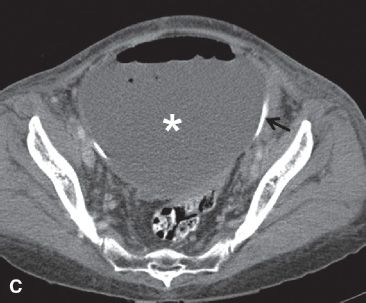
FIGURE 29.6. A 53-year-old woman with recurrent papillary serous carcinoma of the ovary with serosal bowel implants undergoing treatment with intraperitoneal chemotherapy presents with abdominal pain, vomiting and fever. A: Upright abdominal radiograph demonstrates large amount of free intraperitoneal air with air-fluid level due to the presence of ascites fluid (arrows). Multiple dilated air-filled loops of bowel are noted. Intraperitoneal chemotherapy infusion catheter is in place with port in left upper abdomen. These findings are compatible with perforated viscus. B: Axial contrast enhanced CT scan in the same patient demonstrates a large amount of free intraperitoneal air (white arrow) and ascites fluid (black arrow). There are multiple dilated loops of bowel. There is a mottled gas collection in the right lower quadrant posterior to the cecum (open white arrow). At surgery, the cecum was found to be perforated. C: Large loculated fluid and gas-containing collection in the pelvis is compatible with an abscess (*). The chemotherapy infusion catheter is visualized at the posterior wall of the abscess (black arrow).
Bacteremia
Most hematogenous bloodstream infections in adult neutropenic cancer patients are due to coagulase-negative Staphylococcus, viridans streptococci, E. coli, Pseudomonas aeruginosa, and S. aureus observed in prospective case-controlled study (24). In patients with solid-organ cancer, E. coli and coagulase-negative Staphylococcus account for nearly 40% of bacteremia, whereas Pseudomonas, S. aureus, and enterococcal bloodstream infections each account for 10% or less (25). Klebsiella species, other Enterobacteriaceae, and S. maltophilia are a serious concern and may become prominent bloodstream infections in certain geographic regions.
Cancer patients with bacteremia who present with extensive tissue involvement/infection are significantly less likely to respond to antimicrobial therapy (26). Other factors associated with poor prognosis in bacteremic adult patients with an underlying malignancy include shock, infection due to multidrug-resistant bacteria, and Pseudomonas and Clostridium species infection (26).
Bacteroides and Clostridium species are the most frequent cause of anaerobic bacteremia, which is most frequently seen in patients with abdominal and pelvic malignancy (26,27). In cancer patients with nonsporulating anaerobes bacteremia, B. fragilis, and Fusobacterium are isolated more frequently compared with Peptostreptococcus and Eubacterium species (28). Polymicrobial infections are more frequent in patients with anaerobic bacteremia compared with aerobic bacterial infections. E. coli, Pseudomonas, Klebsiella species, and among gram-positive cocci, Streptococcus, Enterococcus, and Staphylococcus epidermidis may accompany anaerobic bloodstream infection (28). Concurrent candidemia is uncommon in patients with bacteremia due to anaerobic bacteria, unless patients have (a) high Candida colonization index, (b) indwelling intravascular catheter, (c) are receiving care in critical care unit, or have (d) poorly controlled diabetes, (e) parenteral nutritional support, or (f) severe and prolonged treatment-induced neutropenia. Over 90% of patients with nonsporulating anaerobic bacteremia may also have a deep-tissue abscess (28). The authors suggest that all patients with gynecologic malignancy who present with anaerobic bacteremia be thoroughly evaluated for an infected abdominopelvic or pelvic collection, which may not be clinically apparent at the time of initial presentation.
Hematogenous Clostridium species infection is often seen in patients with gastrointestinal or genitourinary cancer, and acute leukemia (29). Nearly one-third of patients with bacteremia with Clostridium species alone and nearly half with polymicrobial infection present with septic shock (29). Clostridium perfringens is a common species, and Clostridium septicum is uncommon, albeit serious C. septicum infections are seen in patients with intra-abdominal neoplasms (29,30). Diffuse, rapidly spreading cellulitis involving the abdominal wall, groin, and upper thigh area, gas gangrene, and acute intravascular hemolysis indicate the possibility of clostridial infection (29). Early appropriate therapy remains critical in improved response and outcome for patients with deep-tissue anaerobic infection with or without accompanying bacteremia.
Febrile Neutropenia
The organisms associated with infections in patients with neutropenia are shown in Table 29.3. Pseudomonas spp. is a well-recognized cause of serious systemic infections in febrile neutropenic patients. The other nonfermentative gram-negative bacteria such as S. maltophilia have emerged and present as life-threatening pneumonia, bacteremia, or deep-tissue infection; these infections are commonly resistant to standard broad-spectrum antipseudomonal antibiotics, and despite appropriate therapy response may remain suboptimum (17). Patients with peripheral absolute blood neutrophil counts of less than 500 have significantly higher risk of serious infection, especially if the duration of neutropenia is more than 5 to 7 days (1,2). During the first 5 days of neutropenia, bacterial infections are prominent; if neutropenia extends beyond a week, invasive candidiasis becomes a concern; and in patients with greater than 2 weeks of profound neutropenia invasive mold infections such as aspergillosis may be occasionally encountered. In most patients with gynecologic malignancy, isolation of a mold even from sterile samples does not indicate invasive fungal disease; in patients with no known established predisposing factors (31), these saprophytic molds usually represent colonization or laboratory contamination (32).
Catheter-Related Bloodstream Infection
In patients with solid-organ cancer, nearly 70% of gram-positive bacteremias are associated with an infected indwelling vascular catheter; similarly, 60% of gram-negative bacteremias are also related to an infected catheter, whereas only 19% of gram-negative bacteremias in patients with hematologic cancer is due to an infected catheter (33). Coagulase-negative Staphylococcus remains the most commonly isolated microorganism in blood cultures drawn from an indwelling central venous catheter and is frequently regarded as catheter-related bloodstream infection. Similarly, S. aureus, including MRSA bacteremia, may result from an infected indwelling catheter, and successful therapy requires selection of antimicrobial agents that penetrate the biofilm and are effective against the sessile nongrowth stationary phase of the bacteria (34). S. aureus bloodstream infections are associated with high rates of complications (35). Patients with solid-organ cancer with catheter-related S. aureus bacteremia have a fivefold higher probability of developing intravascular complications such as septic thrombosis and less commonly infective endocarditis (35).
Microorganisms Commonly Associated with Infection in Neutropenic Patients |
Gram-Negative Organisms
Escherichia coli
Klebsiella spp.
Pseudomonas aeruginosa
Stenotrophomonas maltophilia
Enterobacter spp.
Gram-Positive Organisms
Staphylococcus spp. including vancomycin-tolerant organisms
Staphylococcus aureus
Coagulase-negative Staphylococcus such as S.epidermidis and S. saprophyticus
Beta-hemolytic streptococci, group A, B, G
α-Hemolytic streptococci (viridans streptococcus spp.)
Enterococcus spp. including vancomycin-resistant strains
Fungia
Candida spp. including C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis
a Patients receiving multiple courses of fluconazole therapy or prolonged antifungal suppression or prophylaxis are at higher risk for infections due to drug-resistant C. glabrata and C. krusei.
Patients with gynecologic malignancy have a higher rate of postoperative infection, catheter malfunction, and unplanned catheter removal following external intravascular catheters compared with subcutaneous/implantable venous access devices (36,37). Nearly one-third of all intravascular catheters were associated with delayed complications in this cancer population; bacteremia was significantly more common in patients with nonimplantable (Hickman catheters) compared with implantable indwelling intravenous devices such as infusaport or peripheral access system ports (38).
IMPLANTABLE DEVICE INFECTIONS
Peritoneal, hepatic, and pleural implantable devices for delivering chemotherapy or drainage of recurring malignant effusions have been successfully used in the last 2 decades. These devices are well tolerated; major complications including bowel perforation are uncommon, and serious infections are seen in less than 5% of cases (39–44). Overall, infections are seen in less than 20% of cases. While serious infections including pocket infections are seldom noticed, when infections such as these do occur, they should be treated aggressively, requiring prompt removal of the infected reservoir and appropriate systemic antimicrobial therapy (39). Abdominal pain and chemotherapy-related discomfort remain as the major problems with these chemotherapy infusion devices.
Urinary Tract Infection
Patients with gynecologic cancer have an increased risk for urinary tract infection. The factors that promote infections are listed in Table 29.4. Nearly one-third of patients, while undergoing pelvic radiotherapy for gynecologic cancer, may have symptoms of urinary tract infection at the onset of therapy or develop infection during the course of radiation therapy (45). Despite appropriate therapy, infections may recur and require several courses of antibiotic therapy (45). Patients who have undergone pelvic exenteration remain at increased risk for ascending urinary tract infection (14); recurrent pyelonephritis in these patients leads to an increased risk of scarring and permanent renal damage. Older patients with advanced gynecologic and urologic malignancies are also recently being considered for pelvic exenteration surgery (46). In these and other patients with lower renal reserves, a severe episode of pyelonephritis may precipitate irreversible renal failure prompting prolonged renal replacement therapy. We suggest that in selected high-risk patients, preemptive antimicrobial therapy or even secondary antibiotic prophylaxis be considered after an episode of urinary tract infection, especially pyelonephritis.
Factors Promoting Urinary Tract Infections |
Tumor Obstruction
Retrograde urine flow leading to ascending pyelonephritis
Stagnation in patients with outlet obstruction and abscess formation
Instrumentation
Cystoscopy-related introduction of pathogens
Dilatation of strictures
Foreign Body
Urethral stent
Percutaneous nephrostomy tube placement/replacementa
Surgical drains for extended duration
Radiotherapy
Inflammation of bladder mucosa
Suppression of local innate cellular and a cellular immune response
Surgery
Urinary diversions
Organ resection
Lymph node dissection
Anastomotic leak
Chronic surgical wound breakdown
Chemotherapy
Neutropenia
Suppression of local immune surveillance and response to bacterial invasion
Mucosal damage and disruption
a Patients undergoing routine replacement of percutaneous nephrostomy tubes may develop transient bacteremia in the event of prior colonization.
ANTIMICROBIAL AGENTS
It is important to refer to the spectrum of local and regional drug resistance among commonly encountered pathogens in selecting appropriate antimicrobials, especially when treatment is initiated empirically or in patients awaiting microbiologic drug susceptibility results.
SURGICAL ANTIMICROBIAL PROPHYLAXIS
Operative Site
Guidelines for the administration of antibiotics to uninfected patients undergoing pelvic surgical procedures were first proposed by Ledger et al. in 1975 (47). Prophylactic antibiotics are indicated for women undergoing pelvic surgery for malignancy. There have been few studies evaluating this prophylaxis in such cases. Recently, cefazolin was showed to be inferior to cefotetan as a single-dose prophylaxis in women undergoing elective abdominal hysterectomy (48). A placebo-controlled trial showed that using a broad-spectrum cephalosporin plus beta-lactamase inhibitor significantly reduced the risk of major operation-site infection from 27% in patients not given antimicrobial prophylaxis, whereas none given antibiotic developed operative-site infection (49).
The overall postoperative infection rate has been as high as 46%, and surgical-site infection is seen in nearly one-fourth of patients (50). The risk of postoperative infection is adversely affected by prolonged duration of surgery for >5 hours, presence of remote infection at the time of surgery, and duration of hospitalization for longer than 3 weeks; in patients with all 3 risk factors the relative risk of infections increases to 7.3% compared with 3% in patients who have only one of these risk factors (50). Risk factors that have been associated with an increased incidence of infection in patients undergoing surgery for reproductive tract neoplasms include lower socioeconomic status, preoperative colonization, failure to administer perioperative heparin, obesity, advanced patient age, a longer operative period, and a longer hospital stay prior to surgery (51,52). Extent of surgery plays a central role in predicting probability and severity of postoperative infections (12–14,52). However, these risk factors were not uniform among investigators’ evaluations.
Clinical Investigation
The overall operative-site infection rate ranges from 6.7% to 44%. Interestingly, patients with preoperative cervical colonization did not have an increase in postoperative complications including duration of hospitalization, operative time, or febrile episodes compared with patients in whom no cervical colonization was demonstrated (53). An overall decrease in significant postoperative infections was reported in retrospective studies among patients undergoing radical hysterectomy (54,55). The benefit, however, was thought to be associated only with reduced local wound infections (56).
Prospective data have not resolved this issue. Numbers of patients in comparative arms have been small, and the data may, therefore, be inadequate for detecting a statistically significant infection rate. Rosenshein et al. noted no significant difference in overall operative-site infection after preoperative prophylaxis (57), an observation shared by Marsden et al (58). In contrast, significantly lower incidences of infection following preoperative antimicrobial prophylaxis were reported by Sevin et al. (59) and Micha et al. (60). Sevin et al. reported that a short course (3 doses) of prophylaxis was as effective as a long course consisting of 12 doses in preventing major infection after radical hysterectomy (61). If separate operative-site data from prospective studies are combined, the overall incidence of pelvic infection and wound infection was significantly reduced by the administration of prophylactic antimicrobials.
A cost-benefit analysis of 3 doses of cefazolin antimicrobial prophylaxis showed a 29% reduction in infectious morbidity following vaginal hysterectomy and an 18% reduction in postoperative infections in patients following abdominal hysterectomy (62).
One patient population that has a significant surgical incision breakdown is that of women undergoing radical vulvectomy. Anecdotal experience indicates the principal pathogens to be S. aureus and S. epidermidis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree