Fig. 1
Plain lateral radiograph of the forearm demonstrating a large soft-tissue mass (red arrows) and osseous changes including reactive periosteal changes (blue arrow)
Magnetic resonance imaging (MRI) is often the most useful imaging modality for assessing soft-tissue lesions. T1-weighted sequences depict anatomy with exceptional clarity and help define the extent of the tumor and the involvement of or encroachment into adjacent structures (Fig. 2a). Fat-containing lesions appear bright on T1-weighted imaging, which may be helpful in assembling a differential diagnosis. T2-weighted and other water-sensitive sequences are helpful in identifying pathology as well as edema or high-water content (Fig. 2b). Heterogeneity is often appreciated on T2-weighted sequences as it is on post-contrast T1-weighted fat-suppressed imaging. Heterogeneity is a concerning finding, with enhancement indicative of vascular flow and non-enhancing areas potentially reflective of necrosis (Fig. 2c, d). These findings often are found in the setting of a malignant tumor and may be helpful in that it can guide the biopsy to ensure that viable tissue is sampled. MRI may also identify adjacent lymphadenopathy. In general, the MRI images are essential for surgical planning, identification of tumor extension, and involvement of adjacent structures, such as nerves, vessels, tendons, and bone. This additionally permits for a more informed conversation with the patient and their family regarding expectations, functional outcomes, and reconstructive possibilities. Whenever possible, the MRI should be obtained prior to biopsy, as even a needle biopsy can induce edema and bleeding within the lesion. These will, in turn, distort MR images and limit the study’s interpretation and utility.
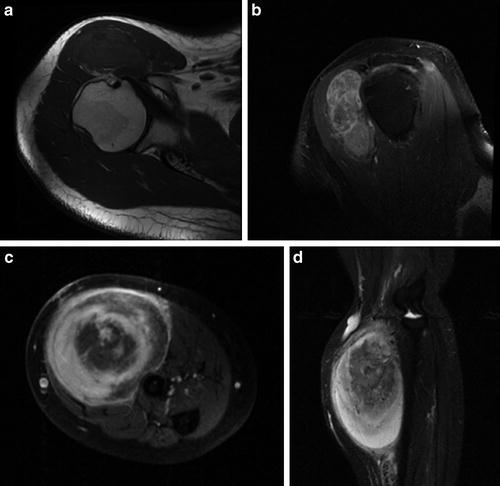

Fig. 2
(a) A T1-weighted axial MR image of the shoulder demonstrating a heterogeneous soft-tissue mass anterior to the humeral head with signal characteristics mostly isointense to muscle. (b) A T1-weighted fat-suppressed post-contrast sagittal oblique MR image of the shoulder demonstrating a lobular soft-tissue mass exhibiting heterogeneous enhancement. (c) A T1-weighted fat-suppressed post-contrast axial MR image of the forearm demonstrating an intramuscular soft-tissue MPNST with extensive enhancement and central areas of necrosis. (d) A T1-weighted fat-suppressed post-contrast sagittal MR image of the forearm demonstrating the large intramuscular MPNST and its relationship to adjacent osseous and vascular structures
Other imaging modalities exist, including Computed Tomography (CT), ultrasound, and positron emission tomography (PET). CT has less utility in the setting of a soft-tissue lesion and, with rare exception, offers no benefit over an MRI. It is rarely required in the context of a soft-tissue mass. Ultrasound may be useful for distinguishing a fluid-filled lesion from a solid lesion and can identify Doppler flow; however, it offers little information regarding either anatomic extent or tumor characteristics. Given its user-dependent variability, it is not regarded as being very helpful for diagnosis or management. Its greatest value is probably that it is noninvasive, inexpensive, and quick. It is reasonable to use as a screening study to confirm that a lesion is in fact more complicated than a cyst. However, since malignant soft-tissue masses may have large cystic components, careful consideration should be given prior to drawing definitive conclusions from an ultrasound study. PET and PET-CT are often utilized for staging purposes and can be obtained to identify areas of disease that may otherwise go unnoticed. The modality uses a radiolabeled glucose analogue to identify areas that are metabolically active. Although controversy exists as to whether quantitative PET standard uptake values (SUVs) or SUV-based derivatives are a reliable indication of malignancy, findings on PET scan may indicate otherwise unknown metastatic foci and can help direct additional diagnostic studies.
Biopsy
The biopsy is the most important aspect of the diagnostic workup, allowing for histologic classification and in turn both prognostication and treatment planning. Unlike many carcinomas in which a fine-needle aspiration can be used to identify cytologic aberrations, sarcomas often require a much larger sample for proper diagnosis. The larger sample is needed to appreciate tissue architecture, staining patterns, and infiltration into surrounding tissue, as well as to appreciate subtle differences between different areas within the tumor. While an open biopsy has slightly higher diagnostic yield, a core needle biopsy performed by an experienced clinician participating in a multidisciplinary sarcoma service can often obtain adequate tissue, and this technique is widely accepted. Alternatively, there is an increasing need for tissue samples in order to pursue basic science and translational research endeavors. Toward this end, an open biopsy permits for collection of larger samples and should be considered if tumor banking is feasible.
Regardless of whether an open or a core needle technique is utilized, the biopsy should be performed in accordance with accepted tenets of sarcoma management. Specifically, esmarch use is contraindicated, though elevation of the limb and use of a tourniquet is permissible. The biopsy tract should be maintained within a single compartment to facilitate complete excision a later time. Neurovascular structures need to be avoided. The biopsy should be performed either by the same individual who will ultimately perform the definitive surgery or by a sarcoma team member after discussion with the surgeon regarding the panned surgical approach. The biopsy should be placed in line with the planned definitive resection incision in order to allow for resection of the biopsy tract. The incision should be extensile, which in the extremity is almost invariably longitudinal. Incisions with transverse, oblique, curvilinear, and other complicated patterns should be avoided with rare exception. Careful attention to hemostasis is essential to avoid hematoma formation, local contamination, and subsequent tumor spread. Drain placement, when required, should be close to and in line with the incision, and similarly, sutures should be close to the skin edges to facilitate complete resection of all involved tissue at the time of definitive resection. It is important to obtain tissue for permanent pathology, cytogenetics, and, in many instances, cultures. Additionally, the biopsied lesion should be reviewed under frozen section at the time of biopsy in order to confirm that representative tissue has been obtained. While some diagnostic information can be gleaned from the frozen section evaluation, its accuracy is recognized as inferior to that of permanent paraffin-embedded histologic review (Athanasian 2002). For this reason, it is often preferred to defer treatment until after permanent slides have been assessed and a final diagnosis has been officially rendered.
An incisional biopsy indicates that the tumor is sampled by removing only a small portion of the lesion’s volume. Alternatively, an excisional biopsy is defined as removing the entire lesion, often with a surrounding cuff of normal tissue. Excisional biopsies are indicated in cases where the lesion is relatively small in size and where the surgical difference between an incisional and excisional procedure is minimal. Although there is no clear quantitative cutoff value, an incisional biopsy is often undertaken for lesions measuring less than 3 cm in diameter (Athanasian 2004). If the additional surgical resection results in meaningful morbidity, an excisional biopsy is probably not appropriate.
Staging
Staging is the single-most relevant determinant of overall survival. It is frequently performed following histologic confirmation of a sarcoma. This avoids unnecessary diagnostic procedures, which is relevant in terms of cost, radiation exposure, and resource utilization. Because most metastatic sarcomas will disseminate hematogenously, the lungs are the primary sites of distant disease. A chest CT offers the greatest resolution with which to identify thoracic disease. It may be obtained in conjunction with a PET scan, which has the added benefit of surveying other potential soft-tissue sites. Although the exact role of PET continues to evolve, it is increasingly being utilized in the context of soft-tissue sarcoma evaluation. If utilized, it is important to obtain a whole-body study, which in some institutions is referred to as a “melanoma protocol.” This ensures the entire body will be imaged, rather than limiting the study to the torso. Finally, a sentinel node biopsy (SNB) may be relevant, particularly in the context of epithelioid sarcoma, rhabdomyosarcoma, clear-cell sarcoma, and synovial sarcoma. SNB is a technique utilized to identify lymphatic spread of tumor, whereby technetium-99 m-labeled sulfur colloid is injected in close proximity to the lesion of concern. Once scintigraphic imaging localizes radioactive uptake in the axilla, an intraoperative gamma probe can identify the involved lymph node, and its subsequent surgical removal can ensue. Additionally, a blue dye, such as methylene blue, can be injected prior to surgery, visually aiding radioactive localization. For the few histologic subtypes that have a predilection for lymphatic spread, SNB is required for proper prognostication and treatment strategies. For the majority of soft-tissue sarcomas, staging is typically reported in accordance with the guidelines set forth by the American Joint Committee on Cancer, which is based upon histologic grade, tumor size, tumor location, nodal dissemination, and/or distant dissemination. A tumor’s histologic type is based upon inherent features defined by microscopic morphologic appearance, immunohistochemical staining, and identification of known cytogenetic aberrations such as translocations, deletions, and trisomies. The grade, which is an important component of tumor stage, is determined based upon a variety of features including tumor differentiation, mitotic activity, and necrosis. Depending upon the grading system utilized, other features such as cellularity, pleomorphism, histologic subtype, and location may be considered. Tumor grade determines the likelihood a tumor will ultimately metastasize, making it an important prognostic indictor and relevant for therapeutic management. Although not as important as surgical margins, it has been, in some reports, predictive of local recurrence.
Surgical Treatment: General Principles
In general, there is no therapeutic role for nonoperative management of soft-tissue sarcomas. A nonoperative course may rarely be elected in the case of advanced and widely disseminated disease, in which local control is neither needed for palliative nor curative purposes. Conversely, local control may offer better quality of life for patients with large, fungating, and ulcerated lesions, and surgery may be considered despite the presence of metastatic disease. Such scenarios are fortunately very rare within the pediatric population.
Surgery remains the mainstay of treatment for localized soft-tissue sarcoma. In such instances, surgery is undertaken with a curative intent. Historically, this has been realized via ablative procedures such as amputations and disarticulations. This radical approach has evolved over time, with the realization that wide excisions offered equivalent survival outcomes to those of amputations, albeit with slightly higher local recurrence rates (Williard et al. 1992). Given the tremendous implications of losing an upper extremity, most surgeons and patients prefer limb-salvage surgery whenever oncologically feasible. However, functional outcome should never take priority over oncologic cure, and properly prioritizing these goals in cooperation with the patient is essential. Overall, limb-salvage surgery is appropriate in approximately 90 % of patients with extremity soft-tissue sarcomas (Lewis et al. 1997), and this figure is probably somewhat higher within the pediatric population. Fingers, and to some extent the hand, differ in that limb salvage is frequently not possible owing to their small size, limited muscle mass, and lack of compartmentalization. Additionally, functional outcomes following finger or ray resections can be quite excellent, making this anatomic region an exception to the rule.
In general, the most important aspect of the surgical resection is the margin. Sarcoma surgery necessitates complete extirpation of the tumor, which by definition means removal of the entire lesion en bloc together with a cuff of surrounding normal tissue. The cuff should serve to completely envelope the tumor, thereby avoiding exposure and tumor spillage as well as removing any microscopic satellite tumor cells. Although important, qualitative margin assessment is subject to interpretation and is arguable, and for these reasons, it is less useful in assessing outcomes. Quantitative margin measurement is a standard component of the final pathology report; however, controversy exists as to what exactly constitutes an adequate negative margin. On the other hand, a positive margin has been widely reported to increase local recurrence. Local recurrence, in turn, portends a worse prognosis both in terms of metastatic dissemination and subsequent overall survival (Gronchi et al. 2010). This finding persists despite the use of adjuvant radiation (Al Yami et al. 2010), which primarily aids in local control.
Example Surgical Procedure: Wide Excision of Upper Arm Tumor
Surgical management should be undertaken by a sarcoma specialist within the larger infrastructure of a sarcoma center. This allows for coordinated multidisciplinary care, which brings together the required diagnostic, oncologic, surgical, reconstructive, and rehabilitation needs necessary for management of these immensely complex conditions. Additionally, having the support of experienced nurse practitioners, social workers, child-life caregivers, dieticians, and other team members allows for comprehensive attention not only for the patient but their family members as well.
Proper surgical management begins well before surgery. Sarcoma centers will typically utilize a multi-disciplinary tumor board conference, at which time the diagnosis, the imaging studies, and the proposed surgical and medical management can be discussed among all involved team members. This promotes a clear understanding of the clinical entity and related relevant issues and hopefully cumulates in an optimal, uniform, and patient-specific management approach. A subsequent discussion with the patient and their family regarding the tumor board discussion is essential. Because a sarcoma diagnosis and its subsequent treatment often have tremendous implications, it is important that the family and, whenever possible, the patient have a clear understanding and realistic expectation regarding the goals and anticipated outcomes. Unrealistic discrepant expectations can lead to physician distrust and poor patient-physician rapport and can undermine future treatment efforts.
Coordination of operative care is also very important. Often, multiple services will collaborate to effect a successful resection and reconstruction. It is not uncommon for an orthopedic oncologist to collaborate with other surgeons such as a hand surgeon, a vascular surgeon, and a microvascular plastic surgeon. For proximal arm or shoulder tumors, in which access to the subclavian vessels is required or for which a chest wall resection is anticipated, a pediatric cardiothoracic surgeon should also be included. Additionally, coordination with the anesthetic team regarding patient position, access needs, expected blood loss, and postoperative pain expectations can be extremely helpful. Reviewing the various perioperative and operative milestones maximizes cooperation, efficiency, and outcomes.
Surgery should be performed in an operating theater that is appropriately sized and staffed for the various surgical teams and their respective equipment. Ensuring that all required equipment is available requires advanced notice to and communication with the operating theater and should not be taken for granted.
Following administration of anesthesia and placement of all required intravenous lines, a Foley catheter should be placed if needed. Many pediatric intensive care units are latex-free environments, and as such latex-free catheters should be used. Careful attention should be paid to the patient’s positioning and padding. Complex surgeries may last many hours. Vigilance is needed to avoid iatrogenic injuries relating to neck or limb positioning or the development of decubitus ulcers secondary to poor padding. Prior to the start of surgery, a “time-out” should be called, confirming proper patient, laterality, procedure, and consent and to clarify any surgical or anesthetic concerns. It is reasonable to identify for the supporting staff critical surgical period during which shift or staff change may not be appropriate. Preoperative and intraoperative antibiotic requirements should be reviewed, and, lastly, a designated team member should confirm that blood products are available if their use is anticipated.
Draping should be wide, allowing for clear visualization of the involved limb. Additionally, it should allow for proximal access to vascular structures in the event of excessive bleeding. The incision should be marked out in keeping with expected preoperative planning. In particular, the tumor’s dimensions should be well known from preoperative MR imaging. The size of the incision and its location need to be carefully considered in order to allow for adequate exposure and to avoid unroofing of the tumor. The incision is marked out after considering the palpable or visible tumor mass, the known radiographic size of the lesion, and the size and location of the biopsy incision.
The surgical incision should ellipse any previous biopsy tract, maintaining this tract together with the en bloc tumor specimen (Fig. 3a, b). Incisions, as a general rule, should be extensile, which typically means longitudinal. Careful attention to hemostasis is required, particularly in cases involving proximally based tumors, where use of a tourniquet is precluded. The surgical dissection should progress in an orderly fashion though dermis, subcutaneous tissue, and fascia. Assuming the tumor resides within the arm musculature, careful dissection around the lesion can now begin. Any neurovascular structures at risk but deemed salvageable based upon preoperative imaging should be traced and protected throughout the procedure. Because large tumors tend to distort normal anatomy by displacing adjacent structures, surgical aids such as a nerve stimulator and a sterile Doppler can help identify and confirm neurovascular structures, thereby minimizing inadvertent iatrogenic injury.
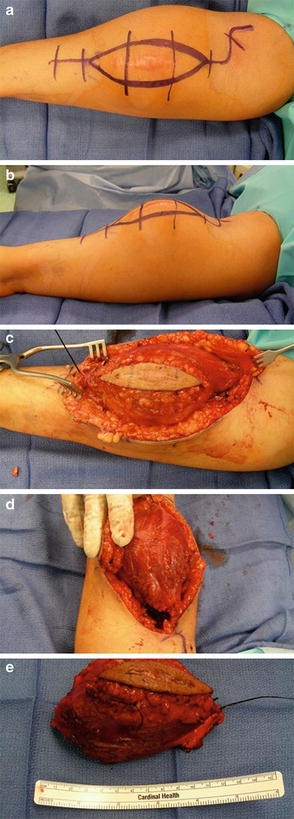

Fig. 3
(a, b) The surgical incision is extensile and planned to incorporate the previous biopsy tract entirely. (c) Medial and lateral dissection proceeds while maintaining a normal cuff of muscle overlying the tumor at all times. (d) A distal pole is delivered, thereby defining the deep surgical plane and allowing for proximal dissection using gentle upward traction. (e) The specimen is tagged with two sutures of varying lengths to aid in its orientation for the purposed of macroscopic and microscopic evaluation
As surgery progresses, the tumor becomes increasingly palpable. It should not be exposed at any time during the procedure, and a normal cuff of tissue should remain encasing it at all times. This requires frequent tactile and visual assessment of both the tumor and the proximate critical structures. Care must be taken to handle the tumor gently to avoid unroofing and exposing the tumor, which in turn may theoretically seed the surgical bed with microscopic disease.
Once medial and lateral (or anterior and posterior) surgical planes are created (Fig. 3c), it becomes helpful to identify, develop, and deliver one pole of the lesion (Fig. 3d). This identifies the deep plane and allows for gentle upward traction of the specimen. Longitudinal dissection through the deeper plane can now ensue. Ultimately, the entire specimen should be freed from surrounding tissue and thereafter oriented with at least two sutures to assist the surgical pathology team in understanding how the tumor resided within the surgical bed (Fig. 3e). The surgeon should carefully inspect the specimen to assess where margins may be close or positive. Ideally, the surgeon should deliver the specimen to the surgical pathologist to guarantee accurate understanding of the specimen’s orientation and to address any surgical or margin-related concerns that may be relevant to the specimen’s evaluation.
Frequently, intraoperative frozen sections can be obtained from either areas of concern or the peripheral extent of the surgical bed. This can offer added intraoperative reassurance that no overt residual disease remains, but it is admittedly subject to and limited by sample error. If postoperative radiation is indicated, 4–6 metallic clips should be placed at the peripheral extent of the surgical bed to facilitate simulation and guide radiation therapy planning. Hemostasis should be obtained, and the wound should be generously irrigated. If soft-tissue reconstruction is not required and a large defect exists, judicious use of drains is important to avoid a postoperative collection. Careful attention to closure is important to avoid excessive tension on the soft-tissues. Given the frequent use of adjuvant radiation for large high-grade soft-tissue sarcomas, additional insult to the soft tissues increases the risk of wound dehiscence and postoperative wound complications. Postoperative splinting should be considered to allow for soft-tissue rest; however, careful attention should be paid to ensure it is well padded and not excessively tight.
Adjuvant Therapy
The role for conventional chemotherapy in low-grade sarcomas is extremely limited and would largely be utilized in the setting of metastatic disease. Small (<5 cm) localized intermediate and high-grade lesions can usually be successfully managed with wide surgical resection alone, and the addition of adjuvant conventional chemotherapy has marginal benefits at best. Larger high-grade tumors (>10 cm in size) are very likely to disseminate, and in such instances, use of adjuvant chemotherapy should be strongly considered. Cases in which metastatic disease is identified warrant its use, recognizing that it is largely intended for palliation at this point. To some extent, the use of conventional chemotherapy is a histology-specific consideration, with some tumors being more chemoresponsive than others.
The use of adjuvant radiation, either preoperatively or postoperatively, has been shown to improve local control for large high-grade sarcomas. Typical indications for adjuvant radiation include a high-grade sarcoma exceeding 5 cm in size or in the case of a smaller tumor that was removed without adequate surgical margins. There may be an extended role for use of radiation in cases of small hand tumors completely excised where local recurrence could result in a subsequent amputation; however, this would require careful patient-specific consideration.
Usually administered at equivalent doses ranging between 50 and 66 Gy, radiation therapy combined with surgery offers improved local control rates compared with those following surgery alone (Pisters et al. 1996; Yang et al. 1998) and is routinely used to treat residual microscopic disease, improving local control rates beyond that of tumor bed resection alone (Rosenberg et al. 1982). Despite one report demonstrating substantial improvement, it is less frequently employed in the setting of low-grade tumors and in these cases surgery remains the mainstay of treatment .
Select Histologic Subtypes
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in the pediatric population, making up approximately 7 % of pediatric malignancies (Ognjanovic et al. 2009). It alone accounts for nearly 40 % of pediatric soft-tissue sarcomas. The alveolar subtype (ARMS), comprising 20 % of all RMS cases, is usually a sporadic event, encountered in an older adolescent pediatric population and frequently involves the extremities. Although rare familial syndromes have been associated with RMSs, these findings are more common in very young patients, making it less relevant to the alveolar RMS population.
Histologically, RMS is a small round blue cell tumor. Because the differential diagnosis of small blue round blue cell tumors includes numerous malignancies such as lymphoma, Ewing’s sarcoma family of tumors, mesenchymal chondrosarcoma, and small cell osteosarcoma, additional diagnostic evaluation is critical. ARMS is characterized by closely packed round cells separated by regions that appear morphologically similar to pulmonary alveoli (Fig. 4). By definition, if more than 50 % of the lesion demonstrates this pseudoalveolar pattern, it is classified as ARMS. Immunohistochemistry can be helpful in identifying muscle markers such as desmin, muscle-specific actin, myoglobin, and Myo-D. Myogenin, in particular, is associated with alveolar RMS (Dias et al. 2000) and independently portends a worse prognosis (Heerema-McKenney et al. 2008). Electron microscopy can aid in identifying characteristics inherent to rhabdomyoblasts such as myofilaments and Z-band material. Molecular studies, using RT-PCR, can demonstrate defined translocations including t(2;13)(q35;q14) and t(1;13)(p36;q14), which result in the chimeric fusion genes PAX3-FKHR and PAX7-FKHR, respectively. Aside from aiding in the diagnosis of ARMS, these findings also impart clinical and prognostic information. Patients presenting with metastatic disease tend to be older and tend to fare far worse if they exhibit the PAX3-FKHR fusion genes than if they exhibit the PAX7-FKHR fusion gene (Sorensen et al. 2002).
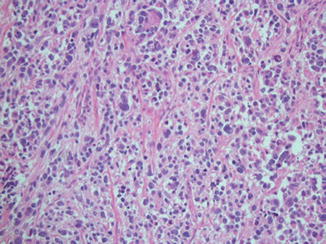

Fig. 4
Low-power (10×) hematoxylin and eosin staining of an alveolar rhabdomyosarcoma characterized by packed round cells within a pseudoalveolar region
Treatment for RMS has evolved since the 1970s through the enrollment of patients in six large cooperative trials involving over 5,000 patients. As a result, survival rates have improved overall from 20 % to 70 %, considering all histologic subtypes. These studies have culminated in a complex algorithmic treatment schema that considers multiple tumor- and patient-specific variables. The current prognostic stratification scheme builds upon and incorporates the clinical grouping classification instituted by the Intergroup Rhabdomyosarcoma Study Group (IRSG) and the subsequently used tumor, node, and metastasis (TNM) staging system. This algorithm largely assigns patients to chemotherapeutic regimens involving either the two-agent regimen consisting of vincristine and actinomycin D or the three-agent regimen including vincristine, actinomycin D, and cyclophosphamide. Additionally, it aids in identifying which patients require radiation therapy. It does not delineate surgical management, which remains a patient-specific matter guided by the general understanding that complete resection is preferable, but that microscopic tumor may be left to avoid excessive functional morbidity. Amputations are not generally indicated. Sentinel node biopsy is essential, both for prognostication and for treatment guidance.
Overall survival for alveolar RMS is somewhere in the center, ranging from 55 % to 76 % estimated 3-year failure-free survival (Raney et al. 2001). Although this is probably more reflective of disease biology and efficacy of adjuvant therapy, it is recognized that patients undergoing either complete resection or resection of all gross disease (IRSG clinical groups I and II) enjoy improved outcomes compared with patients undergoing subtotal resections or those with metastatic disease (IRDG clinical groups III and IV) (Neville et al. 2000). The utility of a second-look surgery and that of surgical metastasectomy remain unclear in the context of RMS and offer no obvious benefit at this point in time .
Synovial Sarcoma
Synovial sarcoma (SS) is the second most common soft-tissue sarcoma in the pediatric population after RMS (Andrassy et al. 1998). It is an intermediate- to high-grade malignancy that accounts for up to 10 % of soft-tissue sarcomas overall. Of these, 30–50 % of cases have been reported in patients below 20 years of age (McCarville et al. 2002; Okcu et al. 2003), and approximately 30 % of pediatric cases occur in the upper extremity (Stanelle et al. 2013). Despite its name, the tumor does not typically arise within or involve synovial tissue, though it does overwhelmingly develop in the extremities and, as such, frequently arises in para-articular regions. Although pain may be a presenting complaint, many tumors persist for extended periods of time, exhibiting a seemingly unremarkable and indolent course.
Histologically, SS is characterized as being either monophasic, biphasic, or poorly differentiated. The monophasic subtype is composed of relatively uniform-appearing spindled cells (Fig. 5a). The biphasic subtype contains both spindled and epithelial cells, which create nests or cords (Fig. 5b). The poorly differentiated subtype can have a variety of morphologic appearances, including that of a small-blue-round-cell tumor (Fig. 5c). Immunohistochemistry demonstrates frequent keratin and epithelial membrane antigen (EMA) positivity. A number of other immunohistochemical markers have been identified as being variably positive, including TLE1, CD99, and S100. The tumor is recognized as having a typical chromosomal translocation, t(X;18) (p11;q11), present in over 95 % of cases (Sandberg and Bridge 2002) that results in either a SYT-SSX1, SYT-SSX2, or less commonly SYT-SSX4 fusion gene product. While the identification of the translocation can be very helpful in confirming the diagnosis, the clinical significance of the specific gene product is currently less clear. While multiple prognostic variables such as mitotic rate, p53 expression, and Ki-67 index have been proposed to date, their value remains controversial. Conversely, tumor size has emerged as a prognostic indicator, with localized tumors larger than 5 cm disseminating in over 50 % of cases and resulting in death in up to 37 % of patients (Lewis et al. 2000).
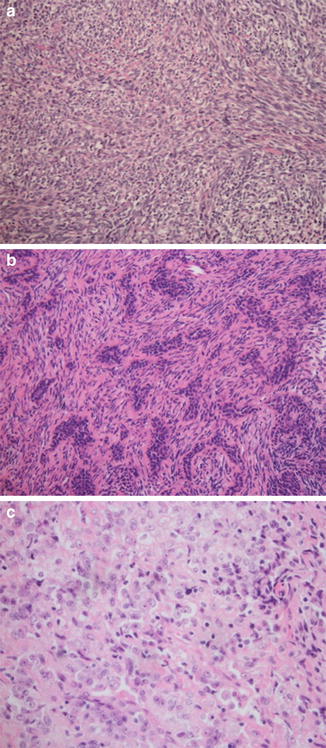

Fig. 5




(a) Low-power (10×) hematoxylin and eosin staining of a monophasic synovial sarcoma composed of monotonous uniform spindled cells. (b) Low-power (10×) hematoxylin and eosin staining of a biphasic synovial sarcoma composed of nests of plump epithelial cells admixed within a background of spindled cells. (c) Low-power (10×) hematoxylin and eosin staining of a poorly differentiated synovial sarcoma composed of small round cells, without spindled or epithelial features
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


