Fig. 1
X-ray showing an osteosarcoma arising from the proximal humerus with large soft tissue mass
Plan x-rays can also provide valuable information when evaluating soft tissue tumors. For example, the presence of phleboliths seen on x-ray would be consistent with an arteriovenous malformation. The presence of bone erosion would be suggestive of an aggressive soft tissue tumor; scalloping and deformity of the bone would be suggestive of a slow-growing and probably benign tumor (Lodwick et al. 1980).
Magnetic Resonance Imaging (MRI)
MRI is the most useful imaging modality when evaluating soft tissue tumors. It can help visualize the tumor and its surrounding inflammatory zone, as well as differentiate it from the surrounding fat and muscle tissue. It can also delineate the neurovascular structures and bone, helping the surgeon to decide preoperatively which structures can be preserved or sacrificed during surgical resection of the tumor. The accuracy of MRI scans done after surgery (e.g., after marginal excision of unsuspected malignant tumors) is greatly affected by the postsurgical changes; hence MRIs should be done before any biopsy or surgical procedure (Davies et al. 2004; Kaste et al. 2002; Puhaindran et al. 2010).
When MRIs are used to evaluate bone tumors, they can show the intramedullary and soft tissue extent of the tumors and will demonstrate the presence of skip metastases within the bone (Fig. 2). The involvement of surrounding neurovascular bundles, one of the important criteria for deciding if limb salvage surgery is possible, can also be accurately assessed with MRI.
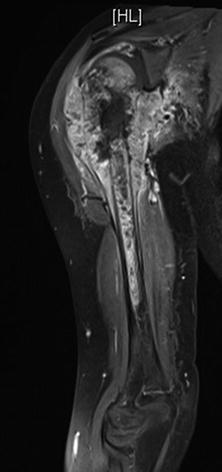

Fig. 2
MRI showing the extent of intramedullary involvement as well as the large soft tissue component of the tumor that has invaded intro the glenohumeral joint
Computed Tomography (CT) Scans
CT scans of the chest are used to stage the disease in patients with bone and soft tissue sarcomas, since this is the most common site for distant metastases. This provides valuable information on the prognosis for patients and helps determine if surgical resection of any metastases identified is possible. CT scans of the abdomen and pelvis should be considered for staging in patients with advanced melanomas and squamous cell carcinomas.
CT scans of the affected limb are not as useful as MRI scans in delineating the local extent of the soft tissue tumors and bone tumors. However, they can be helpful in providing a more detailed assessment of the bone structure in bone sarcomas, aiding in surgical planning and reconstruction.
The benefit of the use of CT scans in evaluating children with cancer needs to be balanced against the risk of radiation exposure. This is especially so in very young children and when used to evaluate patients after treatment when looking for tumor recurrence.
Nuclear Medicine Scans
Technetium-99 bone scans are used for staging in patients with bone sarcoma. They can be used to look for bone metastases and synchronous and skip lesions for bone sarcomas. These scans are not specific, since “hot spots” detected on bone scans are due to increased bone turnover. These can be caused by tumors, recent trauma, or infection.
Positron emission tomography (PET) scans are increasingly used when staging patients with soft tissue sarcomas, bone sarcomas, and advanced melanomas (Roberge et al. 2012; Charest et al. 2009). They allow assessment of the whole body, including the regional lymph nodes, the lungs, and other extrapulmonary sites of metastases. PET scans can also be helpful in differentiating between benign and malignant tumors in patients with neurofibromatosis (Benz et al. 2010). They can also be used to evaluate the response of rhabdomyosarcomas, osteosarcomas, and Ewing sarcomas to treatment (Piperkova et al. 2009).
Imaging studies are generally not required for patients with thin melanomas <1 mm in thickness. Though there is no consensus on the best imaging modality for staging in melanoma, imaging should be considered for staging purposes in patients with melanomas >1 mm in thickness. Choices in these patients include ultrasound, CT scans, or PET scans. For patients with nonmelanoma skin cancers like squamous cell carcinomas or basal cell carcinomas, local imaging with x-rays or MRI can be considered for large tumors if deep invasion is suspected. CT scans can be considered when evaluating patients with enlarged draining lymph nodes found during clinical examination.
Principles of Biopsy
The biopsy is the final step in obtaining the diagnosis. Though biopsies may appear to be simple procedures, they need to be carefully planned. A poorly performed biopsy can result in the need for a larger resection or even amputation of the limb that could have been avoided with proper planning and execution (Mankin et al. 1982, 1996). The exact technique of biopsy used depends on the size and location of the mass, as well as the experience of the surgeon and the pathologist within the institution. Options for biopsy include needle biopsies and open incisional and open excisional biopsies. In addition to biopsy of the primary lesion, bone marrow biopsies may also be required for staging purposes in patients with Ewing sarcoma and rhabdomyosarcoma.
Needle Biopsies
Needle biopsies are the least invasive of the biopsy procedures. They can be performed quickly in the outpatient setting under local anesthesia or under imaging guidance for deeper lesions. However, this may be more challenging for young children, who may not be able to tolerate needle biopsies under local anesthesia, and sedation or even general anesthesia may be required.
Core biopsies are generally preferred to fine needle aspirations in the diagnosis of soft tissue and bone tumors, since the architecture of the tumor can be seen in the core samples, in addition to the tumor cells. However, even core biopsies may at times not provide sufficient tissue for a satisfactory diagnosis, since immunohistochemistry and even molecular studies may need to be done to confirm the diagnosis.
Like surgical biopsy procedures, there is a risk of complications like hematoma formation and infection associated with needle biopsies. Seeding of tumor cells along the biopsy track has to be assumed, and this has to be taken into account when planning the biopsy and performing the final surgery.
Open Biopsies
Many factors need to be considered prior to performing an open biopsy. For example, contamination of the biopsy track with tumor cells following a biopsy will occur. It is for this reason that the incision should be placed longitudinal to the limb and along the incision that will be used for the definitive resection. This will allow that the entire track can be excised during the resection. Transverse or Bruner-type incisions should be avoided. The approach to the tumor should also be the most direct possible, with minimal blunt dissection and exposure of adjacent structures like nerves and vessels. In the upper limb, it is advisable to perform the biopsy in a bloodless field under tourniquet, and meticulous hemostasis is required to prevent hematoma formation, since that will allow the seeding of tumor cells. Suggested approaches for biopsy in the upper extremity, and the areas where there is a high risk of contamination of vital neurovascular structures, are included in Figs. 3 and 4 (Athanasian 2007).
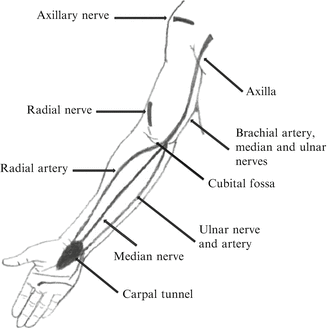
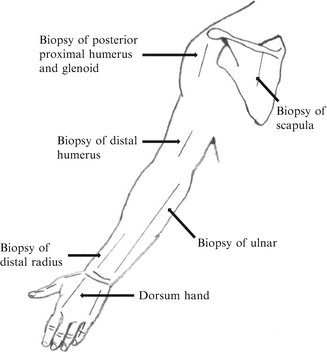

Fig. 3
Diagram showing areas of potential contamination during biopsies of the upper extremity

Fig. 4
View from the back showing suggested incisions for biopsies in the upper limb
Incision biopsies are often the most appropriate technique for suspected malignant soft tissue and bone tumors. Some tissue should be sent for frozen section analysis to ensure that adequate tissue from the lesion has been sampled before the wound is closed. Definitive treatment should be differed till final analysis of the tissue is done since it is often difficult for the pathologist to make a definitive diagnosis on frozen section. For skin ulcers that are thought to be due to malignancy, the biopsy should be taken from the periphery of the ulcer.
Excision biopsies involve complete removal of the tumor around its capsule, through the reactive zone of the tumor. This should be avoided for suspected malignant soft tissue tumors, since there is a high chance of incomplete excision of the tumor with contamination of the entire surgical bed. Excisional biopsies should, however, be considered for suspected melanomas and shave and punch biopsies avoided if possible. This is because an excision biopsy will allow the entire lesion to be examined, and the depth of invasion can be properly ascertained for the specimen.
Skin Tumors
Nonmelanoma Skin Cancers (NMSCs)
The majority of nonmelanoma skin cancers (NMSCs) are basal cell carcinomas (BCC) or squamous cell carcinomas (SCC). The development of these tumors is strongly associated with exposure to ultraviolet radiation, and often they develop after decades of overexposure. As a result, these cancers are relatively rare in children, and are seen in those with underlying predisposing causes, and much more common in adults (Christenson et al. 2005; Sasson and Mallory 1996).
Melanin is a skin pigment which serves to filter out ultraviolet radiation in the skin, and the risk of developing NMSCs in patients with inherited (albinism) or acquired (vitiligo) absence of melanin is higher than in the general population. Other risk factors for the development of NMSCs include immunosuppression (e.g., transplant patients), previous treatment for cancer and exposure to carcinogens, and xeroderma pigmentosum. Xeroderma pigmentosum is an autosomal recessive disorder that affects the ability of the patient to repair DNA damage caused by ultraviolet radiation. This results in a 1,000× risk in the development of skin cancers, both NMSCs and melanoma. Patients with xeroderma pigmentosum may develop symptoms as early as 18 months of age, when they get sunburn after trivial exposure to the sun. These patients then develop multiple freckles and persistent skin erythema in the sun-exposed areas from 2 years of age and later skin cancers at a median age of 8 years. Patients may also have inflamed eyes that are extremely sensitive to the sun. Avoidance of sun exposure is the best method of prevention and treatment for this condition. The prognosis for this condition is poor, with less than half of the patients with this condition surviving beyond the age of 20 years (Kraemer et al. 1984, 1994).
As mentioned earlier, the actual numbers of BCCs and SCCs seen in children are not known, since they are not reportable conditions, but it is thought that there is an increasing incidence in children.
Basal Cell Carcinoma
Basal cell carcinomas (BCCs) are slow-growing tumors that originate from the keratinocytes of the basal epithelium. Although they tend to be locally aggressive, BCCs rarely metastasize. There are four different subtypes: nodular, superficial, pigmented, and sclerosing. Nodular BCCs are the most common form of this tumor, whereas sclerosing are the most aggressive. Complete excision with negative margins is required for all types of BCCs, and a cure rate of >95 % can be expected.
One of the predisposing causes for the development of BCCs in children is nevoid basal cell carcinoma syndrome (NBCC). It is also known as basal cell nevus syndrome or Gorlin syndrome. NBCC is an autosomal dominant condition that may affect multiple systems including the skin, bones, nervous system, eyes, and endocrine system. The incidence is estimated at one in 50,000–150,000 people. Some of the features include development of odontogenic keratocysts of the jaw, palmar, and plantar pits and the early development of basal cell carcinomas, often under the age of 20 years (Kimonis et al. 1997, 2013). The diagnostic criteria for NBCC include two major or one major and two minor criteria as stated below (Kimonis et al. 1997).
Major Criteria
1.
More than two BCCs or one under the age of 20 years
2.
Odontogenic keratocysts of the jaw proven by histology
3.
Three or more palmar or plantar pits
4.
Bilamellar calcification of the falx cerebri
5.
Bifid, fused, or markedly splayed ribs
6.
First degree relative with NBCC syndrome
Minor Criteria
Any one of the following features:
1.
Macrocephaly determined after adjustment for height
2.
Congenital malformations: cleft lip or palate, frontal bossing, moderate or severe hypertelorism
3.
Other skeletal anomalies: Sprengel deformity, marked pectus deformity, or syndactyly of the digits
4.
Radiological abnormalities: bridging of the sella turcica, vertebral anomalies like hemivertebrae, fusion or elongation of the vertebral bodies, modeling defects of the hands, and feet or flame-shaped lucencies of the hands or feet.
5.
Ovarian fibroma
6.
Medullablastoma
Squamous Cell Carcinoma
Squamous cell carcinomas can present as rapidly growing red nodules that can develop ulceration over time. During clinical evaluation, the size and depth of the lesion should be assessed and the regional lymph nodes examined to see if they are enlarged and palpable. Systemic examination should also be done to assess for distant spread.
Surgical treatment involves complete excision of the tumor with negative margins (circumferential and deep), which can be assessed intraoperatively using frozen section analysis or postoperatively. Generally, margins of 4–6 mm are recommended (NCCN Clinical Practice Guidelines in Oncology for Basal Cell and Squamous Cell Cancers). Reconstruction can be decided upon once complete excision has been achieved, once negative margins have been confirmed on pathology assessment of the resected specimen. Other local treatment options include electrodessication and cryotherapy, or application of topical agents such as 5-flourouracil, though the cure rates for these treatments are lower than those for surgery. If surgery is not an option for a patient, radiotherapy can also be used for local control of the tumor. Enlarged lymph nodes should be assessed with a needle or open biopsy, and should lymph node disease be detected, it has to be addressed either surgically, with a block dissection or with radiotherapy.
Melanomas
Melanomas are aggressive skin tumors that arise from melanocytes and are responsible for 1–2 % of all cancer deaths in the United States. It remains rare in children, though the incidence is increasing. There is often a delay in the diagnosis of melanoma in children, partly due to a low index of suspicion and partly due to the atypical clinical presentation. Up to half of melanomas are amelanotic, while 30 % are nodular (Lange et al. 2007; Ceballos et al. 1995). As a result, modifications to the ABCDE criteria used to assess melanomas in adults (asymmetry, border irregularity, color variation, diameter of more than 6 mm, and evolving) have been proposed. This additional “ABCD,” which includes attention to amelanotic lesions, bleeding and bumps, color uniformity which can be seen in children, and de novo nodules of any diameter, should be kept in mind when assessing children for possible melanomas (Mones and Ackerman 2003; Cordoro et al. 2013; Reed et al. 2013).
In prepubertal children, up to one third of melanomas arise in large congenital nevi (nevi that are larger than 2 % BSA of the child). Up to 50 % of melanomas that arise in large congenital nevi are seen in children below the age of 10 years. It has been reported that the transformation risk for large congenital nevi is between 2 % and 20 % (Sasson and Mallory 1996; Lange et al. 2007; Ceballos et al. 1995; Mones and Ackerman 2003; Cordoro et al. 2013; Reed et al. 2013). In view of this, some authors have advocated early excision of large congenital nevi, both to remove a potential melanoma precursor and for cosmetic purposes (Reed et al. 2013).
Patients with dysplastic nevus syndrome, also known as “familial melanoma syndrome,” are also at increased risk of developing melanomas as early as at 10 years of age. These patients may have a family history of melanoma. Another clue that patients may have dysplastic nevus syndrome is the development of multiple “normal” nevi by the age of 5–6 years. During puberty, these nevi may then evolve further, increasing in size to between 5 to 10 mm, and may also develop indistinct borders and have different colors including pink, brown, and black. There are also various forms of dysplastic nevi, ranging from macular forms to papules with a surrounding lighter macular component. Some of the worrying signs of dysplasia include a macular component that is larger than 6 mm in diameter. Evolution of the nevus, with the development of a new area of black pigmentation, is a possible indicator of malignant change. Melanomas usually develop within the nevi, though they may arise de novo in other unaffected areas of skin in these patients (Sasson and Mallory 1996; Ceballos et al. 1995; Mones and Ackerman 2003; Reed et al. 2013). Hence, yearly screening is recommended from the age of 10 years for patients with dysplastic nevus syndrome.
Other causes for the development of melanoma in prepubertal children include xeroderma pigmentosum and immunodeficiency states. Transplacental transmission of maternal melanoma has also been reported, and there is often multiorgan involvement at the time of birth and the prognosis for these newborns is poor.
In teenagers, the pattern of disease is closer to that seen in adults, with fair skin, increased number of benign nevi, and a history of severe sunburn in childhood associated with an increased risk of developing melanoma. Teenage boys tend to develop melanomas on the face and trunk, while females tended to get it on the lower limbs and hip. Increased use of indoor tanning has been cited as a possible cause for the increase in melanoma incidence among teenagers, and avoidance may be helpful in preventing it.
The pathologic diagnosis of melanoma can be a challenge, because many melanocytic proliferations may have pathologic features that overlap with both benign and malignant conditions (Ceballos et al. 1995; Cordoro et al. 2013; Reed et al. 2013; Averbook et al. 2013). These have been collectively termed atypical melanocytic proliferations (AMPs). When performing a biopsy, it is recommended that an excision biopsy be performed, so that the entire lesion, including its depth, can be completely assessed. An experienced dermatopathologist may be needed to assess these lesions, especially the more difficult lesions. At times, other molecular diagnostic techniques like fluorescent in situ hybridization (FISH) and comparative genomic hybridization (CGH) may be required to help establish the diagnosis.
Surgery with wide excision of the primary tumor is the mainstay of treatment of melanoma. While there are no guidelines for the margins required in prepubertal children, it is felt by some authors that 1 cm margins are sufficient for melanomas of all thickness in this age group (Reed et al. 2013). For teenagers, the margins should follow NCCN Clinical Practice Guidelines in Oncology for Melanoma in adults (NCCN Clinical Practice Guidelines in Oncology for Melanoma).
Thickness (mm) | Recommended margin (cm) |
|---|---|
In situ | 0.5–1.0 |
<1 | 1 |
1–2 | 1–2 |
2.01–4 | 2 |
>4 | 2 |
Wider radial margins of clearance above what is recommended have not been shown to improve outcomes.
While the utility of sentinel lymph node biopsies in helping to prognosticate for adults with melanomas >1 mm is clearly established, its role in prognostication for children is less certain. Furthermore, it is known that nevus cell aggregates can be seen even in benign entities like Spitz nevi or cellular blue nevi. There is also controversy over the significance of positive sentinel nodes in children, whether completion lymphadenectomy of the draining lymph node basin should be done for children with positive sentinel lymph nodes (Reed et al. 2013).
It was previously thought that the outcomes of treatment of melanoma in children were better than that for adults. However, it was realized that this could have been due to the inclusion of benign lesions like Spitz nevi in the case reviews. Controversies still exist in the pathologic diagnosis of melanomas; hence the validity of results reported in previously published case series is uncertain (Ceballos et al. 1995; Cordoro et al. 2013; Reed et al. 2013; Averbook et al. 2013). However, it is generally felt that the prognosis for teenagers is similar to that for adults.
The options for systemic treatment in adults with advanced and metastatic melanoma have expanded greatly. Agents like ipilimumab, vemurafenib, and dabrafenib are now added to interleukin 2 as treatment options for patients with systemic disease (NCCN Clinical Practice Guidelines in Oncology for Melanoma). There are many other agents that have also been shown to be active against melanoma. In addition, immunotherapy with vaccines is also being intensively evaluated for use in melanoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


