Local Anesthetic Solutions for Regional Anesthesia in Infants, Children, and Adolescents
Santhanam Suresh
Charles J. Coté
The use of local anesthetics has increased in the pediatric population because of the rising interest in the use of regional anesthetic techniques by a variety of specialists as a means for providing analgesia (1). To improve both safety and effectiveness the practitioner should have a clear understanding of the pharmacology of local anesthetic solutions. In this chapter, we will review basic mechanisms or local anesthetics, methods to reduce potential toxicity, and new directions for resuscitation should a local anesthetic overdose or unintended intravenous injection occur.
Mechanism of Action of Local Anesthetics
Sodium channels are necessary for the propagation and generation of nerve impulses. Local anesthetics reversibly bind to Na+ channels of the nerve thereby inhibiting the propagation of nerve impulses. Although individual sensory axons and other parts of the nervous system have different types of Na+ channels, the general action of the local anesthetics on these channels is the same (2). The drugs bind to their target sites on the channels in bare nerve membranes rapidly, in about 1 to 10 seconds at the 50% inhibitory drug concentration (IC50) and dissociate in about the same time. The IC50 concentrations are much lower than the clinically injected concentrations, for example, IC50 for channel inhibition by lidocaine is 0.2 mmol per L, but a successful block requires an injection of a 1% solution, almost 40 mmol per L. This is due to the fact that less than one molecule in 20 of the injected dose of 1% lidocaine is found within the nerve during blockade (3). The major reasons for this inefficient delivery system are (a) the protonated drug has a pH of 5 to 6 and thus poorly penetrates the perineural area and (b) the extraneural and intraneural vasculature rapidly removes the local anesthetic from the area around the nerve (4). Sodium channels can be inhibited in two ways: (a) by a conformational mechanism (where the activation gating is suppressed; mainly neutral anesthetics) or (b) by an occlusion mechanism (where the pore is blocked; mainly charged local anesthetics). Most tertiary amine local anesthetics are in dynamic equilibrium between charged and neutral forms; hence, they inhibit Na+ channels through both mechanisms. Two major methods to increase the delivery of local anesthetics to their effect site are (a) to neutralize (alkalinize) the injectate thereby increasing the fraction of uncharged drug molecules to promote perineural penetration and (b) to add a vasoconstrictor such as epinephrine to decrease the rate of vascular removal.
Alkalinization of local anesthetics is often advocated as a means for improving drug delivery to the nerve tissue; however, if the pH exceeds 7, the solution becomes less soluble hence leading to precipitation (5). Therefore, it is crucial not to overneutralize the local anesthetic solution; adding bicarbonate should occur just prior to injection so as to avoid precipitation. This may particularly be a problem with bupivacaine and ropivacaine where a small amount of bicarbonate (0.1 mEq Na HCO3 per 10 mL) may cause precipitation within a matter of minutes (5,6). Conversely, this explains the diminished effectiveness when injecting local anesthetic into areas of infection where tissue acidity causes the local anesthetics to be in a more protonized state and therefore less able to cross biologic membranes. Vasoconstrictors are effective in prolonging a block only for local anesthetics that do not have intrinsic vasoconstrictive properties (e.g., bupivacaine but not ropivacaine) and those that are hydrolyzed by local esterases (e.g., chloroprocaine).
Another very important factor is that hydrophobic local anesthetics more rapidly cross the fat layer of biologic membranes. Thus, the more fat-soluble agents cross into neural tissue more readily (e.g., bupivacaine) than those that are more hydrophilic (e.g., procaine). Another method to speed the onset, prolong the duration of local anesthetic action, and increase the intensity of the block is
to increase the concentration of the drug (e.g., 2% vs. 0.5% lidocaine) (bearing in mind the safe dosing limits, see below). Conversely, if the intended effect of the local anesthetic injection is primarily to produce analgesia (e.g., pure sensory blockade vs. sensory plus motor blockade) a more dilute concentration of local anesthetic solution generally will preferentially block sensory fibers while sparing motor fibers, for example, for postoperative caudal/epidural analgesia or for continuous catheter peripheral nerve blockade. The relative potency of local anesthetics for a variety of clinical applications varies considerably (Table 47.1).
to increase the concentration of the drug (e.g., 2% vs. 0.5% lidocaine) (bearing in mind the safe dosing limits, see below). Conversely, if the intended effect of the local anesthetic injection is primarily to produce analgesia (e.g., pure sensory blockade vs. sensory plus motor blockade) a more dilute concentration of local anesthetic solution generally will preferentially block sensory fibers while sparing motor fibers, for example, for postoperative caudal/epidural analgesia or for continuous catheter peripheral nerve blockade. The relative potency of local anesthetics for a variety of clinical applications varies considerably (Table 47.1).
Table 47.1 Relative Potency of Local Anesthetics | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
Tachyphylaxis
This is a clinical phenomenon whereby repeated injections of the same dose of local anesthetic solution lead to decreasing pharmacodynamic effects. An interesting clinical phenomenon to tachyphylaxis is the dependence on dosing intervals. If the dosing intervals are short enough that no pain is perceived, tachyphylaxis does not occur. Conversely, if the patient’s pain has recurred prior to redosing, tachyphylaxis may be observed. This is thought to be due to a central sensitization (so called “wind-up”) phenomenon (7).
Toxicity of Local Anesthetics Solutions
Toxicity of local anesthetic solutions includes central nervous system (CNS), cardiac toxicity, allergic reactions, and local tissue toxicity (Table 47.2). In general, CNS toxicity manifests as circumoral tingling followed by lightheadedness, dizziness, tinnitus, garrulousness, slurred speech, visual disturbances, and seizures. Cardiac toxicity may manifest initially as hypertension and tachycardia (due to intravascular injection of epinephrine within the local anesthetic solution) followed by vasodilation, hypotension, cardiac arrhythmias, and severe cardiac dysfunction leading to cardiac arrest. Since children usually receive high-dose local anesthetics while under anesthesia the vast majority of symptoms are masked. Electrocardiographic monitoring can be particularly helpful, since doubling or tripling of the amplitude of the T wave and ST-segment elevation often indicate intravascular injection of epinephrine, particularly with bupivacaine (Fig. 47.1) (8). The CNS manifestations of local anesthetic overdose or intravascular injection are readily offset with intravenous benzodiazepines but such administration will not block the cardiac depression. Cardiac depression, particularly with the amides, is very difficult to reverse. During the past several years the successful use of intralipid for the management of local anesthetic toxicity has been reported. The original discovery was based on the observation that an adult with carnitine deficiency seemed to be very sensitive to cardiac arrhythmias induced by bupivacaine (9). Since carnitine is essential for fatty acid mitochondrial transport to the heart the authors postulated that bupivacaine may cause further impairment of fatty acid transport (9). Instead, the authors found the opposite of their postulation. Their laboratory dose escalation experiment with Sprague-Dawley rats that were pretreated or received 10%, 20%, or 30% intralipid compared with saline found a shift in the dose response to bupivacaine-induced cardiac arrest: approximately 18 mg per kg for saline, approximately 28 mg per kg for 10% intralipid, approximately 50% for 20% intralipid, and approximately
82% for 30% intralipid (10). The authors subsequently demonstrated accelerated removal of bupivacaine and recovery from bupivacaine-induced cardiac toxicity in an isolated rat heart model (11). Thus, it appears that intralipid is protective of bupivacaine-induced cardiac toxicity. The authors then performed a large animal study and found that 4-mL per kg of 20% intralipid substantially improved survival from bupivacaine-induced cardiac arrest (12). The premise that lipids can act as a sink to counteract the cardiovascular effects of amide anesthetic solutions has been shown to be very effective in animal experiments (13). Since the publication of these animal experiments a number of case reports of the use of lipid for management of local anesthetic toxicity seem to substantiate the efficacy of this treatment (14,15,16,17). Further investigations suggest that long-chain triglyceride infusions may be more efficacious than medium-chain triglyceride infusions and that recurrence of cardiac toxicity may occur after initial rescue (18,19). One pediatric patient was rescued with 3 mL per kg of 20% intralipid followed by an infusion of 0.25 mL per kg per minute until resuscitation (20). An expanded description of the literature and the evolvement of this therapy can be found on www.lipidrescue.org maintained by the University of Illinois in Chicago. These multiple case reports of successful management of toxicity using intralipid are very encouraging but much further research is needed in terms of determining the most effective dose, concentration, and duration of intralipid infusions (21,22,23,24). Our recommendation is to have intralipid available in any location where a long-acting amide local anesthetic solution is being utilized. The intralipid solution (20% emulsion) should be administered as soon as the local anesthetic toxicity is suspected. A continuous infusion of lipid emulsion is recommended for about 1 hour following exposure to local anesthetic toxicity. Note that this is a guideline based upon individual clinical reports in adults and children and that the most important issue always remains excellent chest compressions to maintain perfusion and pulmonary ventilation to ensure oxygenation while instituting the administration of the intralipid (25). The use of antiarrhythmics such as amiodarone and inotropics to support cardiac function is essential; intralipid should be viewed as an important supplement to the resuscitation (19). Probably the most important safety factor is to always double check the proposed dose (Table 47.3) and the concentration (Table 47.4) of the local anesthetic to be used so as to remain within recommended limits, combine the local anesthetic with low-dose epinephrine where appropriate (Table 47.5) so as to slow the absorption and to have a marker of vascular injection (electrocardiographic changes), and administer the block in graded
doses with multiple aspirations so as to reduce unintended intravascular administration.
82% for 30% intralipid (10). The authors subsequently demonstrated accelerated removal of bupivacaine and recovery from bupivacaine-induced cardiac toxicity in an isolated rat heart model (11). Thus, it appears that intralipid is protective of bupivacaine-induced cardiac toxicity. The authors then performed a large animal study and found that 4-mL per kg of 20% intralipid substantially improved survival from bupivacaine-induced cardiac arrest (12). The premise that lipids can act as a sink to counteract the cardiovascular effects of amide anesthetic solutions has been shown to be very effective in animal experiments (13). Since the publication of these animal experiments a number of case reports of the use of lipid for management of local anesthetic toxicity seem to substantiate the efficacy of this treatment (14,15,16,17). Further investigations suggest that long-chain triglyceride infusions may be more efficacious than medium-chain triglyceride infusions and that recurrence of cardiac toxicity may occur after initial rescue (18,19). One pediatric patient was rescued with 3 mL per kg of 20% intralipid followed by an infusion of 0.25 mL per kg per minute until resuscitation (20). An expanded description of the literature and the evolvement of this therapy can be found on www.lipidrescue.org maintained by the University of Illinois in Chicago. These multiple case reports of successful management of toxicity using intralipid are very encouraging but much further research is needed in terms of determining the most effective dose, concentration, and duration of intralipid infusions (21,22,23,24). Our recommendation is to have intralipid available in any location where a long-acting amide local anesthetic solution is being utilized. The intralipid solution (20% emulsion) should be administered as soon as the local anesthetic toxicity is suspected. A continuous infusion of lipid emulsion is recommended for about 1 hour following exposure to local anesthetic toxicity. Note that this is a guideline based upon individual clinical reports in adults and children and that the most important issue always remains excellent chest compressions to maintain perfusion and pulmonary ventilation to ensure oxygenation while instituting the administration of the intralipid (25). The use of antiarrhythmics such as amiodarone and inotropics to support cardiac function is essential; intralipid should be viewed as an important supplement to the resuscitation (19). Probably the most important safety factor is to always double check the proposed dose (Table 47.3) and the concentration (Table 47.4) of the local anesthetic to be used so as to remain within recommended limits, combine the local anesthetic with low-dose epinephrine where appropriate (Table 47.5) so as to slow the absorption and to have a marker of vascular injection (electrocardiographic changes), and administer the block in graded
doses with multiple aspirations so as to reduce unintended intravascular administration.
Table 47.2 Toxicity of Local Anesthetics | |||
|---|---|---|---|
|
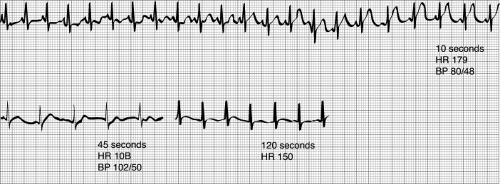 Figure 47.1. Electrocardiographic changes associated with the intravenous injection of bupivacaine and epinephrine 1:200,000. Note the marked increase in the height of the T wave (8). (Reproduced from Freid EB, Bailey AG, Valley RD. Electrocardiographic and hemodynamic changes associated with unintentional intravascular injection of bupivacaine with epinephrine in children. Anesthesiology 1993;79:394–398, with permission.) |
Table 47.3 Maximum Recommended Doses and Duration of Action of Commonly Used Local Anesthetics | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Table 47.4 Local Anesthetic Concentration and Its Conversion to Milligrams Per Milliliter | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Classes of Local Anesthetic Solutions
Local anesthetics are represented by two main classes of drugs, the amino-amides (amides) and the amino-esters (esters) (Table 47.6). The main differences between the two classes are that the amides undergo enzymatic degradation by the liver whereas the esters are hydrolyzed by plasma cholinesterases (26); these differences play an important role in the metabolism and safe use of local anesthetics particularly in the neonate and infant.
Amides
The local anesthetics belonging to this class include lidocaine, bupivacaine, ropivacaine, and levobupivacaine; however, levobupivacaine is not available for commercial use in North America. These are the most commonly used local anesthetics in infants and children. Although the selection of these agents is related to the time of onset and the desired duration of the local anesthetic effect, careful attention must be paid to the potential toxic effects. For example, the ability of the neonate’s liver to biotransform and to oxidize and reduce the local anesthetics is vastly diminished compared with that of the adult (27). At approximately 3 to 6 months of age, the ability to conjugate drugs achieves adult levels (28,29). Another consideration is that older children will absorb local anesthetics more rapidly than do adults and therefore achieve higher blood levels of local anesthetic; for example, higher blood levels have been found in children undergoing intercostal nerve blocks than in adults (30). After caudal administration of local anesthetic solution, peak plasma concentrations are obtained in children and adults in about 30 minutes (31). The steady-state volume of distribution (Vdss) for amides is increased in children compared with adults although clearance (Cl) is similar (31,32). Since the elimination half-life (t1/2) is related to the volume of distribution and clearance as follows t1/2 = (0.693 ÷ Vdss)/Cl, the larger steady-state volume of distribution results in prolongation of the elimination half-life. This may not play an important role in single-dose injections but may play a vital role in continuous infusions or repeated injections. The risk with repeated doses has been demonstrated to be greater in infants and children than in adults (33,34).
Table 47.5 Epinephrine Dilution and Conversion to Micrograms Per Milliliter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Table 47.6 Commonly Used Local Anesthetics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
The systemic absorption of local anesthetics is very dependent upon the site of injection. A common rule of thumb is to remember the nemonic ICE-Blocks (Intercostal > Caudal > Epidural > peripheral nerve Blocks) in order of increased risk for toxicity (30). This relationship is very important to bear in mind since the same dose of local anesthetic when administered as a peripheral nerve block may be toxic when used for intercostal nerve blocks.
Bupivacaine
This is the most commonly used local anesthetic solution in infants and children in North America. The pharmacokinetics as well as the pharmacodynamics has been well studied in infants and children (31,32,35,36,37,38). The average duration of analgesia is about 5 to 6 hours after a single bolus injection (39,40). The concentration of the local anesthetic used depends on the site, the desired density of blockade, postoperative “street readiness,” and the potential for toxicity. The concomitant use of other local anesthetic solutions including infiltration anesthesia has to be taken into consideration before a total dose (mg per kg) of local anesthetic solution is taken into consideration.
Pharmacology
Bupivacaine is bound to α-1-acid glycoprotein. It is a racemic mixture of the levo and dextro-enantiomers. Although the levo-enantiomer is the active form that provides the clinical effect of the local anesthetic solution, the dextro-enantiomer causes the adverse effects related to local anesthesia including cardiac and CNS toxicity. The cytochrome involved with metabolism of bupivacaine is CYP3A4, which may be immature in neonates and infants younger than 6 months, thus accounting in part for the delayed clearance compared with that in older children and the need to reduce total drug exposure, particularly if administered by a continuous infusion (see below) (41,42).



