Leukocytes
Neutrophil Disorders
A diverse group of leukocyte disorders is encountered in newborn infants. Different blood cells are involved (e.g., neutrophils, lymphocytes, eosinophils), and the disorders may be quantitative or qualitative in nature. This section focuses on abnormalities that are particularly relevant to newborn infants because of the frequency with which they are encountered (e.g., neutrophil changes associated with bacterial infections) or because they are unique to this age group (e.g., congenital leukemia or neonatal alloimmune neutropenia).
Normal Leukocyte Count in the Neonatal Period
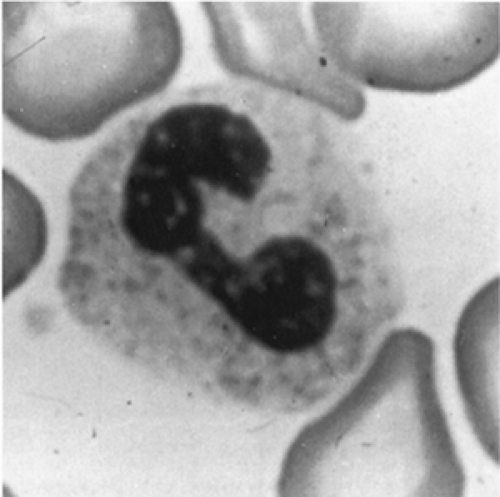
The morphologic definition of a mature neutrophil is a cell in which the nucleus is distinctly segmented into two or more lobes connected by a thin filament. Cells with no lobulation and those in which the width of the narrowest segment of the nucleus is greater than one-third the width of the broadest segment are referred to as nonsegmented neutrophils or bands (Fig. 46-15). During the first two weeks of life for full-term and premature infants, a band to segmented neutrophil ratio greater than 0.3 should be con- sidered abnormal (308). Examination of a peripheral blood smear during the first few days of life characteristically reveals an excess of polymorphonuclear neutrophils. Particularly in premature infants, some immature forms (e.g., promyelocytes, myelocytes) may be seen. Sometime between the fourth and seventh days of life, the lymphocyte becomes the predominant cell and remains so until the fourth year of life.
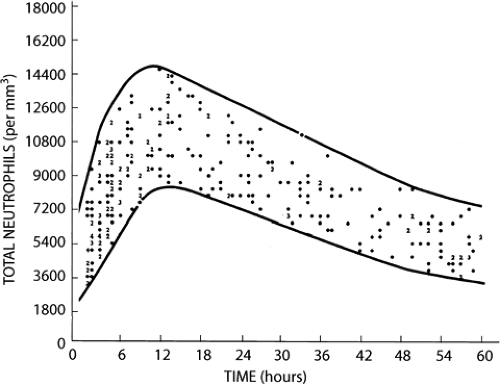
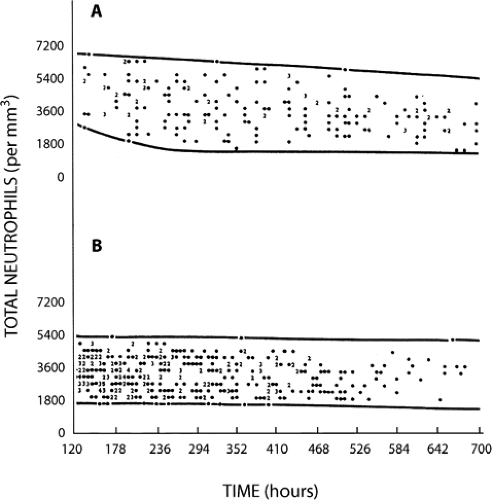
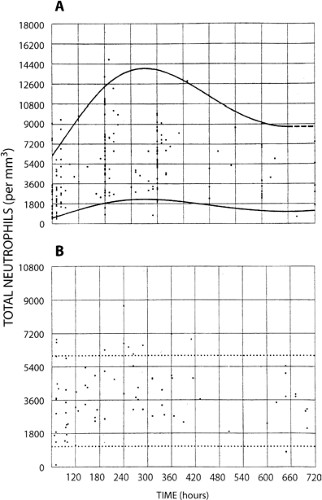
Counts of segmented and band (i.e., nonsegmented, young) neutrophils of full-term and very low-birth weight infants have been reported by a number of investigators (308,309,310,311,312,313 and 314). The lower limit of normal for neutrophil counts in very low-birth weight infants are significantly below those for term infants, and the data most widely used for defining neutropenia in NICUs is that generated from the University of Texas Southwestern Medical Center at Dallas by Manroe (312) and Mouzinho (314) (Figs. 46-16, 46-17 and 46-18).
Although the definition of neutropenia is essentially a statistical consideration based on data obtained from studies of healthy term and premature newborn infants, there is consensus among experts that absolute neutrophil counts above 1,000/μL are not likely to place neonates at risk of acquiring clinically significant infections (315). In our experience it is a helpful rule of thumb to consider an absolute band plus neutrophil count below 1,000/μL to be “clinically significant” in full-term and premature newborn infants.
Physiological neutrophilia is common in neonates in the first week of life. According to Thilaganathan and associates (316) total leukocyte counts in umbilical cord blood ranged between 7.25 to 48 × 109/L with a mean of 13.8 × 109/L. After birth neutrophil counts increase to levels up to 23,000 at 16 hours post labour and then gradually decrease to less than 9.5 × 109/L at 5 days of age.
The mechanism for the physiological neutrophilia seems to be a surge in cytokine secretion. Barak and associates (317) found that serum and urinary colony-stimulating activity levels were significantly increased (3-5 fold) on the first and fourth days of life, but declined to normal values by the 14th and 28th days. Ishii and associates (318) found that G-CSF and M-CSF levels were significantly higher on day 1 after birth and then gradually decreased. Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels did not change significantly during the neonatal period. Interestingly, these investigators found that granulocyte colony-stimulating (G-CSF) and GM-CSF produced in the placenta (trophoblasts and decidual stromal cells) are the major cause of physiological leukocytosis in newborn infants at birth.
Neutropenia
Causes of neutropenia in newborn infants include decreased production of neutrophils, increased destruction, or a combination of both mechanisms (Table 46-11). Most episodes occur during the first week of life and are related to low gestational age, low birth weight, infections, pregnancy induced hypertension, severe neonatal asphyxia, drug therapy, or other perinatal events (319). Late-onset neutropenia, defined as an absolute neutrophil count of
less than 1,500/μL at a postnatal age of more than 3 weeks has been reported in well, very low birth-weight infants with anemia of prematurity, and reticulocytosis (320). It is speculated that a requirement of progenitor cells for enhanced erythropoiesis associated with the physiological anemia of prematurity limits their availability for granulopoiesis leading to a decrease in neutrophil production. This condition should be considered physiological and is not generally associated with an increased risk of infection.
less than 1,500/μL at a postnatal age of more than 3 weeks has been reported in well, very low birth-weight infants with anemia of prematurity, and reticulocytosis (320). It is speculated that a requirement of progenitor cells for enhanced erythropoiesis associated with the physiological anemia of prematurity limits their availability for granulopoiesis leading to a decrease in neutrophil production. This condition should be considered physiological and is not generally associated with an increased risk of infection.
TABLE 46-11 CAUSES OF NEONATAL NEUTROPENIA | ||
|---|---|---|
|
The various causes of neutropenia in neonates are summarized in Table 46-11. The commonest causes include neutropenia associated with infection, neutropenia in premature infants, neutropenia in infants of hypertensive mothers, and allo-autoimmune neutropenia (321). Other causes for destruction or underproduction of neutrophils are rare, and include inherited marrow failure syndromes.
Changes in Blood Neutrophils During Bacterial Infection
Neutropenia frequently occurs in the setting of neonatal sepsis. It can be the cause of sepsis, but more commonly it is a consequence of infection. It is noteworthy that neutrophil function, particularly chemotaxis and phagocytosis, is reduced in newborn infants and may contribute to susceptibility to infection (322). In neonates, clinical signs of infection may be minimal, and the speed of evolution of disease may be rapid. Changes in neutrophil number and appearance are often helpful in the diagnosis of bacterial infections in this age group (310).
Changes in Neutrophil Numbers
In infants with systemic bacterial infection, the total neutrophil count is usually decreased (i.e., neutropenia), but may also be increased (i.e., neutrophilia), or normal. In a study of 24 newborn infants with proven bacterial sepsis (positive bacterial cultures from the blood, cerebrospinal fluid, bladder-tap urine, or peritoneal fluid), neutropenia was observed in five, neutrophilia in three, and normal neutrophil counts in the remaining 16 (309). Although neutrophilia is a relatively nonspecific finding and may occur in conditions other than sepsis, the finding of neutropenia is highly significant in newborn infants and may be the first clue to bacterial infection. In the study of Manroe and associates (312) neutropenia was observed in 77% of neonates with confirmed or suspected bacterial disease. Neutrophilia was absent almost as often in infected infants (42%) as it was present (58%). In a more recent study the frequency of neutropenia in newborn infants, defined by reference ranges established by Manroe (312) and Mouzinho (314), was 8.1% (135/1662 cases) (319). In 65% (41/63 cases) of neonates neutropenia was present on the day of the clinical onset of sepsis. In 13% (8/63 cases) neutropenia developed within 3 days of the onset of sepsis, and in 22% (14/63 cases) neutropenia was present before the clinical onset of sepsis. Seventy-seven % of the neutropenic episodes occurred during the first week of life; in 75% of affected neonates the duration of neutropenia ranged from 0 to 8 days, with 75% having neutropenia for less than 24 hours. In addition to neutropenia, increased numbers of immature neutrophils (band forms) and an elevated band to segmented neutrophil ratio are seen in neonates with sepsis. Although Baley and colleagues (323) did not find the immature:total neutrophil ratio to be of value in the identification of sepsis in neonates others have found the opposite and have stressed the potential clinical importance of hematologic changes in the assessment of sepsis in neonates (309,310). In a study of premature infants with proven bacterial infection 73% (11 of 15) of infants had elevated band counts and a reversed band to segmented neutrophil ratio (310).
Changes in Neutrophil Morphology
In addition to numerical changes in neutrophils, morphological changes may appear. During infection, the neutrophils of newborn infants have increased numbers of Döhle bodies (i.e., aggregates of rough endoplasmic reticulum), vacuoles, and toxic granules (310). Rodwell and colleagues (324) have proposed a hematologic scoring system for use in the early diagnosis of sepsis in neonates (Table 46-12). In their studies the combination of a neutrophil count less than or equal to 500/mm3 and scores more than or equal to 3 identified a poor prognostic group (325)
Neutropenia of Prematurity
The most common example of neutropenia seen in NICUs occurs in premature infants, particularly those of VLBW. The mechanisms underlying postnatal neutropenia in such
infants appears to be a combination of reduced total body neutrophil mass, together with reduced numbers of committed neutrophil precursors in the bone marrow at birth and an inability to increase granulopoiesis in response to sepsis (326). The role of recombinant hematopoietic growth factors, in particular granulocyte colony-stimulating factor (rhG-CSF) and GM-CSF remains controversial. There is now substantial data indicating that intravenous or subcutaneous administration of these growth factors at daily doses of 5 to 10 μg/kg produces significant increases in the level of circulating neutrophils (G-CSF), or both neutrophils and monocytes (GM-CSF), without significant clinical toxicity. The use of GM-CSF was found by one group to be associated with fewer episodes of postnatal neutropenia and septicemia (327), but not by another (328). Results of prospective, controlled randomized clinical trials are summarized in Table 46-13 (327,328,329,330,331 and 332). Although some studies have shown a significant decrease in nosocomial infection rates in treated newborn infants as compared to controls, most studies have failed to demonstrate a significant benefit in overall mortality for treated infants. As a result routine administration of growth factors to extremely low (<1,000 g) or very low (<1,500 g) birth-weight infants at risk for neutropenia and sepsis cannot, as yet, be considered standard of care (333,334,335). Definitive answers regarding the role of growth factors, administered either prophylactically or as part of treatment of infection in newborn infants, must await the results of future prospective, randomized controlled trials with adequate numbers of subjects to answer clinically important end-points such as morbidity, mortality, and cost.
infants appears to be a combination of reduced total body neutrophil mass, together with reduced numbers of committed neutrophil precursors in the bone marrow at birth and an inability to increase granulopoiesis in response to sepsis (326). The role of recombinant hematopoietic growth factors, in particular granulocyte colony-stimulating factor (rhG-CSF) and GM-CSF remains controversial. There is now substantial data indicating that intravenous or subcutaneous administration of these growth factors at daily doses of 5 to 10 μg/kg produces significant increases in the level of circulating neutrophils (G-CSF), or both neutrophils and monocytes (GM-CSF), without significant clinical toxicity. The use of GM-CSF was found by one group to be associated with fewer episodes of postnatal neutropenia and septicemia (327), but not by another (328). Results of prospective, controlled randomized clinical trials are summarized in Table 46-13 (327,328,329,330,331 and 332). Although some studies have shown a significant decrease in nosocomial infection rates in treated newborn infants as compared to controls, most studies have failed to demonstrate a significant benefit in overall mortality for treated infants. As a result routine administration of growth factors to extremely low (<1,000 g) or very low (<1,500 g) birth-weight infants at risk for neutropenia and sepsis cannot, as yet, be considered standard of care (333,334,335). Definitive answers regarding the role of growth factors, administered either prophylactically or as part of treatment of infection in newborn infants, must await the results of future prospective, randomized controlled trials with adequate numbers of subjects to answer clinically important end-points such as morbidity, mortality, and cost.
TABLE 46-12 HEMATOLOGIC SCORING SYSTEM IN NEONATES WITH SUSPECTED SEPSIS | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Less frequent causes of neutropenia in newborn infants as a result of either decreased production or increased destruction are summarized below.
Rare Causes of Neutropenia
Increased Destruction of Neutrophils
Neonatal Alloimmune Neutropenia.
Neonatal alloimmune neutropenia is the neutrophil counterpart of the erythrocyte disorder hemolytic disease of the newborn. Estimates of the frequency of alloimmune neutropenia vary widely, with figures ranging from one in 500 to one in 2,000 newborn infants (336,337 and 338). Alloimmune neutropenia occurs when a mother becomes sensitized to a foreign antigen present on the neutrophils of her infant and is then stimulated to form specific immunoglobin G (IgG) antibody directed against this fetal antigen of paternal origin. Transplacental passage of IgG antibody into the fetal circulation results in accelerated destruction of neutrophils in the reticuloendothelial system with consequent neutropenia. Because neutropenia is the direct consequence of transplacentally acquired maternal IgG, the condition is self-limiting, and neutropenia persists for only a few weeks or months. The severity of neutropenia is influenced by the titer and subclass of the maternal IgG neutrophil antibody, the phagocytic activity of the infant’s reticuloendothelial system, and the capacity of the infant’s marrow to compensate for the shortened survival of antibody-sensitized neutrophils.
Investigation of infants with neonatal alloimmune neutropenia has contributed much to the current knowledge of neutrophil-specific antigens (Table 46-14) (339,340), and the antigen systems most often involved are NA1, NA2, and NB1. In approximately one-half of cases the responsible neutrophil-specific antigen system remains unidentified. Although human leukocyte antigen (HLA) antigens are present on granulocytes, and alloimmunization to these antigens is common in pregnancy, HLA antibodies are not thought to be a significant cause of neutropenia in newborn infants. It appears that maternal HLA antibodies are effectively absorbed by HLA antigens in placental tissue; the antibodies that reach the fetus are neutralized by soluble antigens or weakened by having to react with antigens on various cells distributed in the blood and other tissues. In contrast, neutrophil-specific antibodies cross the placenta without any obstacle and concentrate on the target antigen, which occurs only on the relatively small mass of mature neutrophils.
The clinical course of infants with alloimmune neutropenia is of interest. Neutropenia is usually severe and symptomatic infants may present with delayed separation of the umbilical cord, skin infections, otitis media, or pneumonia within the first two weeks of life. In a review of 19 affected infants reported before 1974, Lalezari and associates (341) found that 12 infants had total absence of circulating neutrophils for at least part of their course. The duration of neutropenia ranged from 2 to 17 weeks, with a mean of 7 weeks. Infections were common, and most were caused by Staphylococcus aureus. Two infants died, one with staphylococcal septicemia and the other with pneumonia and possible meningitis. Whilst most infections in newborn infants with neonatal alloimmune neutropenia are
mild, affected infants with severe neutropenia are at risk for serious bacterial infections, and therapeutic intervention should be considered. In the past, in addition to intravenous antibiotic therapy for infants with suspected or proven infection, therapies included exchange transfusion to remove passively acquired maternal neutrophil antibodies, transfusion with compatible antigen-negative granulocytes harvested from the mother or known antigen-negative blood donors, corticosteroids, and high-dose intravenous immunoglobulin (HDIVIG).
mild, affected infants with severe neutropenia are at risk for serious bacterial infections, and therapeutic intervention should be considered. In the past, in addition to intravenous antibiotic therapy for infants with suspected or proven infection, therapies included exchange transfusion to remove passively acquired maternal neutrophil antibodies, transfusion with compatible antigen-negative granulocytes harvested from the mother or known antigen-negative blood donors, corticosteroids, and high-dose intravenous immunoglobulin (HDIVIG).
TABLE 46-13 RANDOMIZED TRIALS OF RECOMBINANT GROWTH FACTORS (rhG-CSF AND rhGM-CSF) IN NEWBORN INFANTS | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree