Fig. 1
Intrauterine Volkmann’s contracture (Copyright Milan Stevanovic)
Imaging and Other Diagnostic Studies
Planar radiographs are useful to assess fracture healing and alignment as well as any evidence of physeal injury affecting growth. Radiographs of bilateral forearms may be useful in documenting the discrepancies in longitudinal and appositional growth.
Magnetic resonance imaging (MRI) can be used to evaluate the extent of muscle damage within each compartment and may augment clinical examination findings in determining the appropriate surgical treatment plan and provide some prognostic information to the patient and their family. MRI can also help distinguish between Volkmann’s ischemic contracture and “pseudo-Volkmann’s contracture.” In pseudo-Volkmann’s contracture, the involved muscles will retain normal signal quality throughout the majority of the muscle. Muscle entrapment at the fracture site may or may not be visualized.
Magnetic resonance angiography (MRA) or traditional angiography is useful for patients who have severe involvement that are anticipated to need a functional free muscle transfer. This can help to plan the recipient vessel anastomosis site as well as assess perfusion to the hand.
Classification
Several classification systems have been described for Volkmann’s ischemic contracture. There is considerable overlap in these systems. Most are based on the clinical severity of the presentation and are used to help direct the appropriate treatment for the identified disability. Most authors recognize the tremendous variability of the clinical presentations and the subsequent limitations of the classification system (Benkeddache et al. 1985; Seddon 1956, 1964; Tsuge 1975, 1999; Zancolli 1979). Fracture location or etiology of the compartment syndrome may also play a role in the pattern of muscle necrosis.
Seddon was the first to introduce the concept of the ellipsoid infarct involving the muscles of the proximal forearm (Fig. 2). He further described a spectrum of contracture from mild to severe. The mild type responds to splinting with little to no residual sequelae, with the possible recurrence of contracture as a young child grows to maturity. The most severe type was described as a limb, which “apart from its envelope is gangrenous and whose treatment is futile” (Seddon 1964). Between these two extremes, he described three separate patterns of presentation:
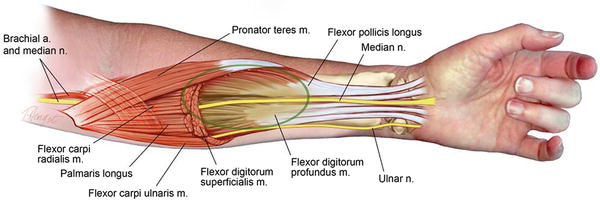

Fig. 2
Seddon’s concept of the elliptical infarct affecting the central portion of the deep compartment of the forearm (Copyright Milan Stevanovic)
Diffuse but moderate ischemia: The main feature in this presentation is contracture. Nerve involvement is limited and resolves spontaneously. Most function returns spontaneously, and at the end of a few months, there is left only residual contracture to correct.
Intense but localized muscle damage: This occurs rarely, but with a distinct presentation in which the primary involvement is that of the deep flexors of the forearm, specifically FDP and FPL. Minimal involvement of the median nerve which is adjacent to the zone of infarction can also be present.
Widespread necrosis or fibrosis: In this presentation there is near-total destruction of all the muscles of the forearm. The median and ulnar nerve function is compromised, and there may be extension of the infarct into the dorsal compartment.
As noted by Zancolli, there is significant variability in the involvement of the hand. His classification system was based entirely on the involvement of the intrinsic muscles (Zancolli 1965, 1979). Type I cases had no intrinsic involvement. In type II deformity, the intrinsic muscles are paralyzed resulting in an intrinsic minus claw hand and opponens paralysis. Type III deformity is defined by intrinsic muscle contracture with the clinical appearance of an intrinsic plus hand and intrinsic plus thumb. In the type IV combined group, there was a mixed presentation of intrinsic involvement. At times there may be some fingers with an intrinsic minus posture (usually the fourth and fifth fingers) with an intrinsic plus posture affecting the adjacent fingers (usually the index and middle). The variability in presentation depends on the ischemic insult and potential recovery to the median and ulnar nerves, and there is no uniform pattern to predict whether the thenar or hypothenar muscles will be spared or extent of the intrinsic paresis or paralysis.
The most commonly used classification system is that of Tsuge (1975). He classified established Volkmann’s contracture into mild, moderate, and severe types, according to the extent of the muscle involvement.
The mild type, also described as the localized type, involves the muscles of the deep flexor compartment of the forearm, usually involving only the flexor digitorum profundus of the ring or middle fingers (Fig. 3). It can involve all the flexor digitorum profundus and the flexor pollicis longus as well. Nerve involvement is absent or mild, typically involving sensory changes which resolve spontaneously. With wrist flexion, the fingers can be fully extended. The majority of mild-type deformities result from direct trauma, such as a crush injury or forearm fracture. They are more typically seen in young adults.
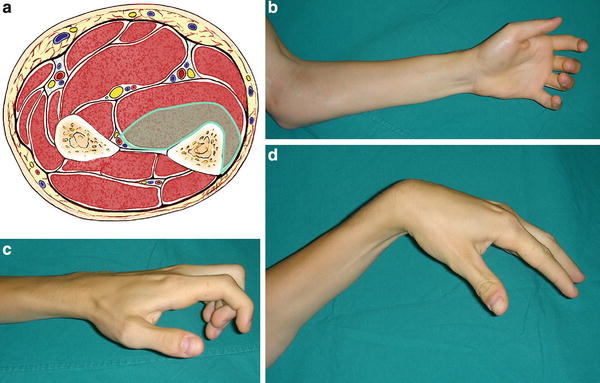

Fig. 3
(a) Cross-sectional representation of Tsuge mild-type Volkmann’s contracture at the mid-forearm level. (b) Volar clinical appearance of mild-type Volkmann’s contracture. (c) Lateral view appearance of mild-type Volkmann’s contracture. (d) Mild-type contracture allows for full extension of the fingers when the wrist is positioned in volar flexion (Copyright Milan Stevanovic)
In the moderate type, the muscle degeneration includes all or nearly all of the flexor digitorum profundus and flexor pollicis longus with partial degeneration of the flexor superficialis muscles (Fig. 4). The wrist is in a flexed position and attempts at wrist extension result in increased flexion posture of the fingers. Neurologic impairment is always present. Sensory impairment is generally more severe in the median than in the ulnar nerve . The hand may demonstrate an intrinsic minus posture due to neurologic impairment. Moderate-type injury was most commonly the result of supracondylar humerus fractures in children between ages five and ten.
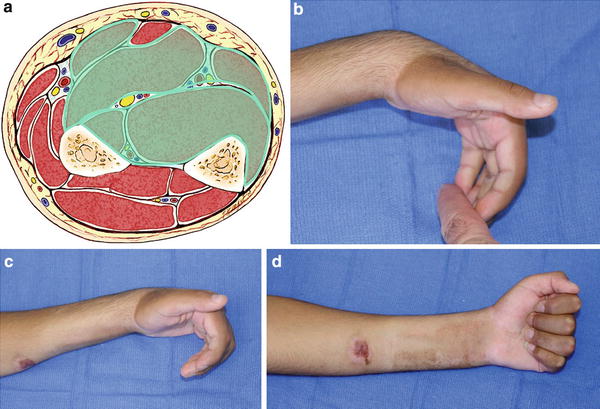

Fig. 4
(a) Cross-sectional representation of Tsuge moderate-type Volkmann’s contracture at the mid-forearm level. (b) Patient with Tsuge moderate-type Volkmann’s contracture. With the wrist in a position of volar flexion, the fingers cannot be brought into full passive extension. (c) Finger flexion contracture is worsened by the wrist being brought into a neutral position. (d) Patient demonstrates full active finger flexion (Copyright Milan Stevanovic)
The severe type involves degeneration of all the flexor muscles of the fingers and of the wrist. There is central muscle necrosis, and varying involvement of the extensor compartment (Fig. 5). Neurologic deficits are severe, including complete palsy of all the intrinsic muscles of the hand. Tsuge categorized those cases which may have had moderate involvement initially, but due to fixed joint contractures, scarred soft-tissue envelope or failed surgeries were categorized as severe. As with the moderate cases, the severe cases were most commonly the result of supracondylar humerus fractures in children.
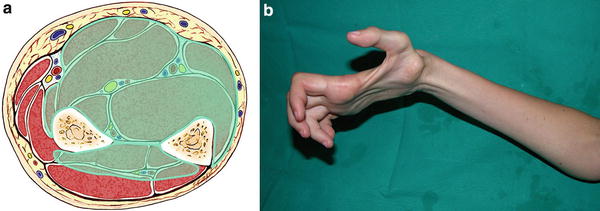

Fig. 5
(a) Cross-sectional representation of Tsuge severe-type Volkmann’s contracture at the mid-forearm level. (b) Patient with severe-type contracture with extensive intrinsic wasting secondary to neurologic involvement at the forearm (Copyright Milan Stevanovic)
Within each classification type, there is a broad range of clinical presentation. This heterogeneity of presentation makes it difficult to apply a specific treatment based solely on classification systems and makes it nearly impossible to provide meaningful outcome and comparison studies.
Outcome Tools
There are no specific outcome tools used to assess preoperative function or postsurgical outcomes for Volkmann’s ischemic contracture. DASH scores may be useful in assessing preoperative and postoperative function, but this scoring system has limited value in the pediatric population. Typically improvement in sensation and function is assessed subjectively or with Semmes-Weinstein monofilament examination which categorizes sensation as normal, diminished light touch, diminished protective sensation, loss of protective sensation, and untestable. Motor function is graded by the British Medical Research Council scoring system, which has limitations.
Volkmann’s Contracture Treatment Options
Nonoperative Management of Volkmann’s Contracture
There is a limited role for nonsurgical management of Volkmann’s contracture. Young age of onset and mild contractures may respond in part or in full to a program of stretching and splinting. For neonatal compartment syndrome, a program of stretching prior to surgical intervention is instituted. Children less than 4 years of age will usually not tolerate splinting (Tables 1 and 2).
Table 1
Volkmann’s Contracture: Non-operative Mangement
Indications | Contraindications |
|---|---|
Mild contracture | Moderate to severe contracture |
Medical conditions precluding surgical intervention | |
Age < 12 months | |
Preoperatively for anticipated surgery to reduce joint contracture |
Table 2
Volkmann’s Contracture: Therapy Recommendations
Splinting |
• Night splinting to maintain wrist and finger extension and thumb abduction extension |
• Removable cast or thermoplast splint |
• Splint with or without surgical intervention through skeletal maturity |
Formal therapy and/or home exercise program (HEP) |
• Directed toward maintaining active and passive range of motion and maintaining supple joint motion |
• Strengthening of residual muscle function |
• Customized splinting as dictated by type and severity of contracture |
Outcomes (Nonoperative Treatment)
There is little data on the nonsurgical treatment of Volkmann’s ischemic contracture. Tsuge noted that in 27 cases of mild contracture, if a program of therapy for stretching and splinting was started within 1 month of injury, “good results can be expected” (Tsuge 1975). Initiation of nonsurgical treatment more than 1 month from the development of contracture was not often effective. No data are provided, nor does the article indicate the number of patients treated nonsurgically.
Operative Treatment
A variety of surgical procedures have been used to treat Volkmann’s ischemic contracture. These have included both bone and soft-tissue management (Table 3).
Table 3
Surgical options for management of Volkmann’s ischemic contracture of the forearm. Late Management of Compartment Syndrome: Surgical options for the forearm
Operation | Authors | Advantages | Disadvantages |
|---|---|---|---|
Bone | Zancolli (1979) | Addresses wrist flexion contracture without causing additional scarring in the forearm | 1. Nonselective shortening of both extensors and flexors |
Proximal row carpectomy | Goldner (1975) | 2. Limb is already shortened from ischemic insult | |
Diaphyseal shortening | Rolands and Lond (1905) | Satisfactory correction of contracture | 1. Nonselective shortening of both extensors and flexors |
Domanasiewicz et al. (2008) | Does not preclude other procedures | 2. Limb is already shortened from ischemic insult | |
Pavanini and Volpe (1975) | 3. High nonunion and refracture rate reported (Domanasiewicz et al. 2008) | ||
Arthrodesis | Botte and Gelberman (1998) | Wrist: Maintains wrist in physiologic position | Wrist: |
Goldner (1975) | If additional tendon transfers are needed, if wrist if fused, one less donor muscle is needed and wrist extensor muscles are available as donors for finger function | 1. Loss of tenodesis effect of wrist position | |
PIP: Fingers maintained in a more functional position. | 2. Decreased grip strength | ||
May be only option to correct intrinsic imbalance | 3. Loss of motion | ||
PIP | |||
1. Loss of finger flexion | |||
2. Resultant loss of grip strength | |||
Soft tissue | Goldner (1975) | Straightforward surgical release of flexion contracture | Loss of active flexion |
Fractional or tendon | More disruption of muscle resting length and loss of flexion strength | ||
Z-lengthening | |||
Infarct excision with tendon reconstruction/transfer | Tsuge advocated infarct excision for mild contractures with only 1–2 finger involvement | Late infarct excision creates more scarring around nerves. Deficit created from scar excision is replaced with more scar tissue later | |
Tsuge (1975) | |||
Flexor pronator slide | Page (1923) | Allows greatest correction of flexion contracture with least impact on muscle resting length | Will not fully correct finger flexion contracture that is not passively correctable in maximal wrist flexion |
Scaglietti (1957) | Wide surgical dissection with potential injury to CIA/PIA | ||
Gosset (1956) | |||
Sharma and Swamy (2012) | |||
Functional free muscle transfer | Only procedure that can restore function for severe (Tsuge classification) contracture | Difficult microsurgical procedure requiring an experienced team | |
Failure can only be remedied with a second functional free muscle transfer | |||
Doi et al. (1993) |
Bone Reconstruction
Skeletal shortening or fusions are frequently performed in conjunction with some of the soft-tissue procedures listed below.
Shortening procedures including shortening osteotomy of the radius and ulna (Rolands and Lond 1905) and proximal row carpectomy (Goldner 1975) have been used to match the skeletal length to the shortened fibrotic muscle. Shortening procedures raise concerns in children, since the forearm is already relatively shortened by the initial ischemic insult to the bone and growth plates. Further, the principal contracture is usually on the flexor surface. Shortening the forearm indiscriminately lengthens the muscle resting length of both the flexor and extensor muscles, neglecting the predominant involvement of the contracture within the flexor compartment. Bony reconstructive procedures for long-standing contractures or for distal reconstruction required for neurologic injury include wrist fusion, trapeziometacarpal joint fusion, and thumb metacarpophalangeal joint fusion. Severe progressive finger deformities can also be managed with arthrodesis in a more functional position. These are ideally done after skeletal maturity but may need to be performed earlier if there is progressive wrist flexion deformity.
Soft-Tissue Procedures
Soft-tissue procedures have included excision of the infarcted muscle, fractional or z-lengthening of the affected muscles, muscle sliding operations (flexor origin muscle slide), neurolysis, tendon transfers , and functional free-tissue transfers, as well as combinations of the above procedures (Chuang 1997; Chuang et al. 1996; Eichler and Lipscomb 1967; Goldner 1975; Gulgonen 2001; Ikuta et al. 1976; Krimmer et al. 1995; Lanz and Felderhoff 2000; Reigstad and Hellum 1981; Seddon 1956, 1964; Sundararaj and Mani 1985; Tsuge 1975, 1999; Zuker 1989; Zuker and Manktelow 2007). Excision of scarred fibrotic nerves without distal function followed by nerve grafting has been described to try and establish some protective sensation in the hand (Hovius and Ultee 2000). Fixed contractures of the joints can be addressed with soft-tissue release including capsulectomy and collateral ligament recession or excision, depending on the joints involved. Fixed contracture of the joints can be surgically addressed simultaneous to management of the shortened muscle.
Preferred Methods
Our preferred methods of treatment depend on the general classification of severity of contracture, individualized to the patient presentation.
Mild (Localized) Type (Deep Flexor Compartment Without Neurologic Deficit)
For mild contractures which have failed to respond to nonsurgical management, our preferred treatment is a muscle sliding operation initially described by Page and subsequently used and endorsed by several others (Lanz and Felderhoff 2000; Nisbet 1952; Page 1923; Scaglietti 1957; Seddon 1956, 1964; Sharma and Swamy 2012; Tsuge 1975). We have found this procedure effective as long as there is clinically good finger flexion. We do not combine this procedure with infarct excision, nor have we found it necessary to release the distal insertion of the pronator teres to correct pronation contracture (Lanz and Felderhoff 2000; Tsuge 1975).
A limited flexor slide may be done for mild deformity, affecting only a portion of the flexor digitorum profundus. In this case, the flexor pronator mass does not have to be released from the medial epicondyle, and the ulnar nerve does not have to be mobilized and transposed. We think that this surgical approach limits potential scarring and vascular compromise to the remaining muscles and nerves in the flexor compartment and that the superficial veins are better preserved in the subcutaneous tissue.
Moderate Type (Deep and Superficial Flexor Compartment with Neurologic Deficit)
For moderate deformity, we still prefer the muscle slide operation to correct the tightness of the flexors, provided that there is still adequate remaining strength in the flexors. Since neurologic impairment is characteristic of the moderate injury, we combine the flexor slide with neurolysis of both the median and ulnar nerves. A separate incision to release the carpal tunnel may also be done.
Depending on the functional deficits, tendon transfer can be combined with flexor slide, either as a staged or simultaneous procedure.
Reconstruction of Thumb Function
Our preferred transfers for thumb flexion are to use brachioradialis or extensor carpi radialis longus to the flexor pollicis longus. Extensor indicis proprius is used for thumb opposition.
Reconstruction of Finger Flexion
When the finger flexors are very weak or absent, a functional free muscle transfer may produce a better functional result than tendon transfers. However, if functional free muscle transfer is not an option, tendon transfers include biceps brachii elongated with autograft (fascia lata or superficialis tendon) to the flexor digitorum profundus. Extensor carpi radialis longus, brachioradialis, extensor carpi ulnaris, and extensor indicis proprius can also be used as donor muscles for reconstruction of finger flexion. These donor muscles do not have sufficient excursion to match the flexor muscles, but in the absence of other options, they can provide adequate improvement in grasp. If flexor carpi radialis or flexor carpi ulnaris are not affected by the ischemic event, these muscles may be used for reconstruction of finger flexion. Similarly, if there is little to no involvement of the flexor digitorum superficialis muscle, this can be transferred to flexor digitorum profundus.
Nerve Reconstruction
When sensory impairment is severe and there has been no recovery, the nerve should be carefully evaluated at surgery. If there is a densely scarred atrophic nerve, resection of the nerve to healthy-appearing fascicles followed by sural nerve graft reconstruction may restore protective sensation to the hand.
Severe Type (Superficial and Deep Flexor Compartments, Extensor Compartment, and Severe Neurologic Deficits)
Severe-type contractures are best treated with functional free muscle transfers (Chuang 1997; Chuang et al. 1996; Doi et al. 1993; Ikuta et al. 1976; Krimmer et al. 1995; Xing-yan et al. 1992; Zucker et al. 1991; Zuker 1989). The donor vessels are usually either the radial or anterior interosseous artery as an end-to-end anastomosis or end-to-side to the brachial artery. The donor motor nerve is the anterior interosseous, which should be resected back to healthy-appearing fascicles. Our preference for the donor muscle is the gracilis . For severe-type contractures with extensive involvement of the extensor compartment, a double free muscle transfer should be considered. As with the moderate type, tendon transfer, nerve graft reconstruction, and late osseous reconstructive procedures may improve final functional outcomes.
Outcomes
Outcomes following Volkmann’s contracture in the upper extremity are difficult to assess. Studies are limited by small numbers of patients, great variability in initial presentations, use of varied surgical techniques, and difficulty in compliance with the long-term follow-up necessary to track patients through skeletal maturity. Ultee and Hovius attempted to provide some information regarding outcomes. They found that all patients who had developed the contracture during childhood had a relatively shortened extremity. Substantial improvements in hand function were noted in those patients who underwent functional free muscle transfer. Tendon lengthening alone often resulted in recurrence of contracture. Finally, in patients who had sufficient remaining muscle, procedures which combined infarct excision, tenolysis, neurolysis, and tendon transfer when necessary produced good hand function (Ultee and Hovius 2005). Sundararaj and Mani noted improvement in sensory function in conjunction with neurolysis. Additional procedures were done simultaneously, and little analysis of outcomes of those other procedures was given (Sundararaj and Mani 1985).
Surgical Techniques
Flexor Pronator Slide
Both volar and ulnar incisions have been described for the surgical approach for a flexor pronator slide (Page 1923; Tsuge 1975). We favor the incision and technique initially described by Page (1923) with some modifications (Fig. 6). The surgical incision begins on the medial distal arm and continues along the ulnar border of the forearm all the way to the wrist. The ulnar nerve is identified and mobilized for several centimeters proximal and distal to the medial epicondyle, including proximal release at the arcade of Struthers. Four to six centimeters of intermuscular septum between the brachialis and triceps is excised to prevent kinking of the ulnar nerve after transposition. The flexor pronator mass is elevated off of the medial epicondyle, taking care to preserve the medial collateral ligament and elbow joint capsule. Inadvertent disruption of the joint capsule is repaired as needed. The origins of the flexor carpi ulnaris, flexor digitorum profundus, and flexor digitorum superficialis are mobilized off of the ulna and interosseous membrane. The dissection is carried out above the periosteum toward the radius. The common interosseous artery arises as a branch of the ulnar artery, crossing the flexor digitorum profundus, where it bifurcates into the anterior and posterior interosseous artery (Fig. 7). The posterior interosseous artery crosses to the posterior compartment at the proximal edge of the interosseous membrane. As this is the dominant blood supply to the extensor compartment, it is important to protect this branch. Working toward the radius, the origin of the flexor pollicis longus is released from proximal to distal. Special attention is needed in this area to protect the anterior interosseous nerve. Throughout the procedure, the wrist and fingers are manipulated to check whether the contracture is improving and to help localize where there is still tightness within the muscle origin. The dissection must often be carried down to the level of the wrist to release adhesions between the flexor tendons and pronator quadratus before full correction is achieved. If necessary, the carpal tunnel should be opened and tendon adhesions released in this area as well. It has been our experience that the lacertus fibrosus and superficial fascia around the antecubital fossa are often tight and a contributor to residual elbow flexion contracture. It can also tether the superficial flexors of the wrist and fingers, contributing to incomplete correction of the wrist and fingers with the muscle sliding only. Release of this fascia helps correct the deformity and also prevents median nerve compression at the level of the pronator. Slight under-correction, which can be addressed by postoperative splinting and rehabilitation, may decrease the reduction in muscle power resulting from the muscle slide. When a pronation contracture is present and not corrected by the release of the flexor pronator origin, we release the pronator quadratus from the distal ulna. Even with a complete release of both pronators and volar distal radioulnar joint capsule, complete correction of the pronation deformity may not be possible due to fibrosis and contracture of the interosseous membrane. At the completion of the muscle slide, the ulnar nerve is transposed to an anterior subcutaneous position. The hand is casted in a position of forearm supination with the wrist and fingers in full extension. Immobilization is continued for a period of six weeks to allow the flexor pronator to heal adequately to its new origin (Fig. 8, Tables 4, 5, and 6).
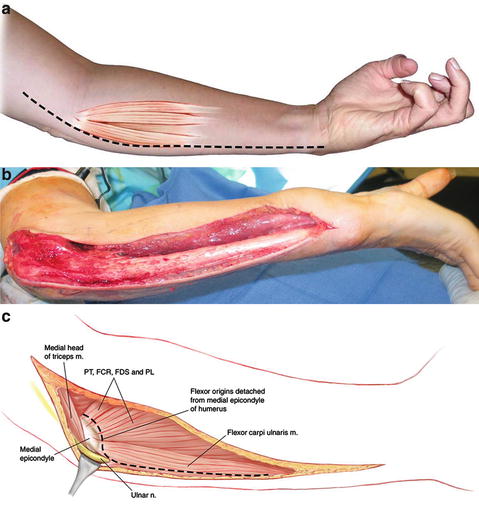
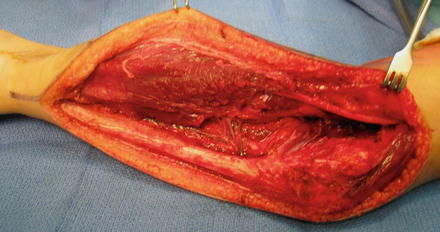
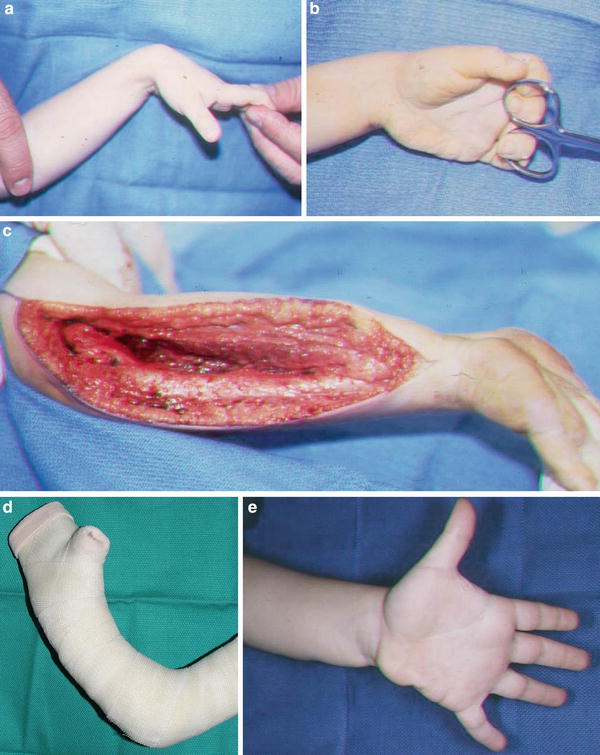
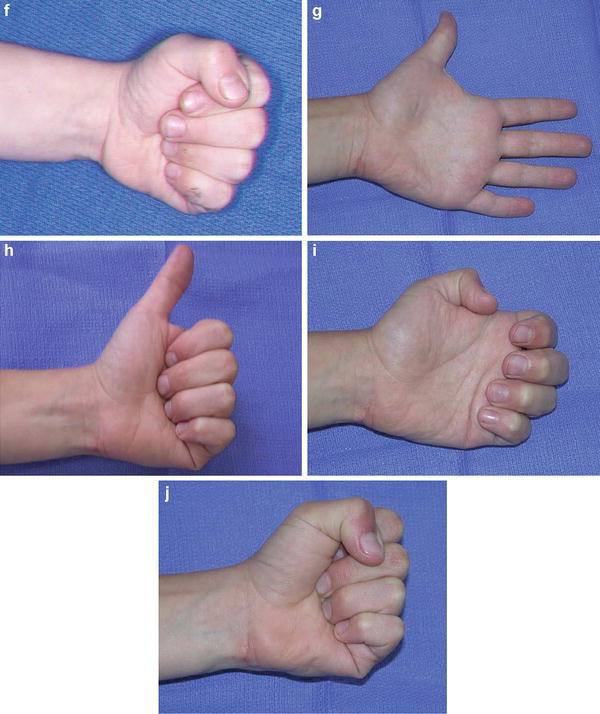

Fig. 6
(a) Diagram of surgical incision for flexor origin slide . (b) Intraoperative surgical incision. (c) Schematic diagram of deeper layer of dissection for flexor origin slide (Copyright Milan Stevanovic)

Fig. 7
Intraoperative appearance of flexor origin slide . The common interosseous artery is seen in the mid-portion of the dissection, dividing into the anterior and posterior interosseous arteries (Copyright Milan Stevanovic)


Fig. 8
This triplet was born with a mild-type neonatal Volkmann’s contracture. At age 4, he underwent a flexor origin slide. He continues to wear night splints to maintain wrist and finger extension. He is now 6 years from surgery and continues to maintain full active wrist and finger extension (Copyright Milan Stevanovic). (a) Preoperative finger motion. Fingers can be passively extended with wrist flexed. (b) Preoperative finger extension with wrist in neutral. (c) Intraoperative contracture release with a flexor origin slide. (d) Postoperative casting with elbow at 90° of flexion, wrist and fingers in full extension. (e) Six-month postoperative extension of fingers and thumb. (f) Six-month postoperative finger flexion, with minimal profundus function. (g) Six-year (age 10) follow-up finger extension. (h) Six-year (age 10) follow-up thumb extension. (i) Six-year (age 10) follow-up showing independent profundus function. (j) Six-year (age 10) follow-up demonstrating composite finger flexion
Table 4




Flexor Pronator Slide: Pre-operative planning
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


