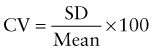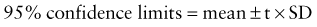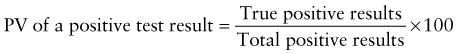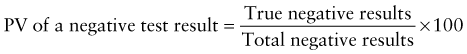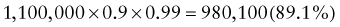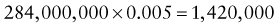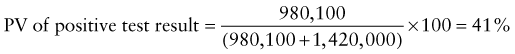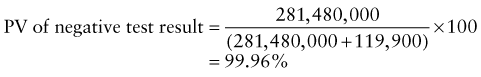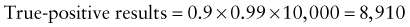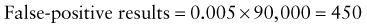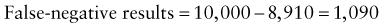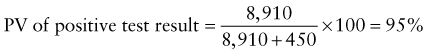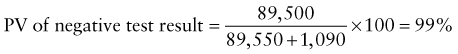Chapter 707 Laboratory Testing in Infants and Children
Reference intervals, more commonly known as normal values, are difficult to establish within the pediatric population. Differences in genetic composition, physiologic development, environmental influences, and subclinical disease are variables that need to be considered when developing reference intervals. Other considerations for further defining reference intervals include partitioning based on sex and age. The most commonly used reference range is generally given as the mean of the reference population ±2 standard deviations (SD). This is acceptable when the distribution of results for the tested population is essentially gaussian. The serum sodium concentration in children, which is tightly controlled physiologically, has a distribution that is essentially gaussian; the mean value ±2 SD gives a range very close to that actually observed in 95% of children (see Table 707-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com ![]() ). However, not all analytes have a gaussian distribution. The serum creatine kinase level, which is subject to diverse influences and is not actively controlled, does not show a gaussian distribution, as evidenced by the lack of agreement between the range actually observed and that predicted by the mean value ±2 SD. In these cases, a reference interval defining the 2.5-97.5th percentiles are typically used.
). However, not all analytes have a gaussian distribution. The serum creatine kinase level, which is subject to diverse influences and is not actively controlled, does not show a gaussian distribution, as evidenced by the lack of agreement between the range actually observed and that predicted by the mean value ±2 SD. In these cases, a reference interval defining the 2.5-97.5th percentiles are typically used.
Table 707-1 GAUSSIAN AND NONGAUSSIAN LABORATORY VALUES IN 458 NORMAL SCHOOL CHILDREN 7-14 YR OF AGE
| SERUM SODIUM (mmol/L) | SERUM CREATINE KINASE (U/L) | |
|---|---|---|
| Mean | 141 | 68 |
| SD | 1.7 | 34 |
| Mean ± 2 SD | 138-144 | 0-136 |
| Actual 95% range | 137-144 | 24-162 |
SD, standard deviation.
Accuracy and Precision of Laboratory Tests
where t is a constant derived from the number of replications. In most cases, t = 2.