A physiologic decrease in circulating creatinine, urea, and uric acid due to an increase in clearance
The average serum creatinine (SCr) during pregnancy is 0.5–0.6 mg/dL
A modest increase in SCr to 1.0 mg/dL, though within normal range, is reflective of kidney impairment
Blood urea nitrogen decreases to approximately 8 mg/dL
An increase in protein excretion to 180–300 mg/24 h is seen in the third trimester because of an increase in filtered load combined with less efficient tubular reabsorption of protein
Normal protein excretion in pregnancy is less than 300 mg per 24 h, with 1+ protein on urine dipstick being abnormal depending on urine volume and concentration
Women with preexisting proteinuria often exhibit an increase in their protein excretion in the second and third trimesters
The usefulness of elevated serum uric acid as a marker for diagnosing preeclampsia is controversial [13]. However, a recent study looking at uric acid in patients with gestational hypertension found it to be an accurate predictor of presence and severity of preeclampsia [14] .
Monitoring uric acid levels and trending them over time can be an important tool to diagnosis.
Definition of Pregnancy-Related Acute Kidney Injury (PR-AKI)
Consensus definitions of AKI in the general population, such as (1) Risk, Injury, Failure, Loss, and End-stage renal disease (RIFLE) criteria; (2) Acute Kidney Injury Network (AKIN) criteria; and (3) Kidney Disease Improving Global Outcome (KDIGO) criteria (http://kdigo.org/home/guidelines/), are extensively used. However, definitions of PR-AKI are not standardized and vary from a slight increase in SCr level to the need for dialysis. The RIFLE criteria focusing on the change in SCr or GFR and urine output has been used in PR-AKI, with more than one prospective study reporting a higher RIFLE stage to be related to unfavorable renal outcomes in women with PR-AKI. High-RIFLE stage also has been shown to have a discriminative power in predicting risk of mortality from AKI in obstetric ICU patients [15, 16]. The Acute Kidney Injury Network (AKIN) criteria is more frequently used to identify PR-AKI [16]. Mayo Clinic reported that most of their obstetric AKI patients had stage 1 AKI by AKIN criteria. Physiologic changes in pregnancy pose a challenge, and more studies are needed to see which criteria are best in PR-AKI.
Newer biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), cystatin C, and complement level have been widely investigated in the nonpregnant population with AKI. To date, none of these biomarkers can be recommended in predicting or diagnosing PR-AKI.
Etiologies of Pregnancy-Related Acute Kidney Injury
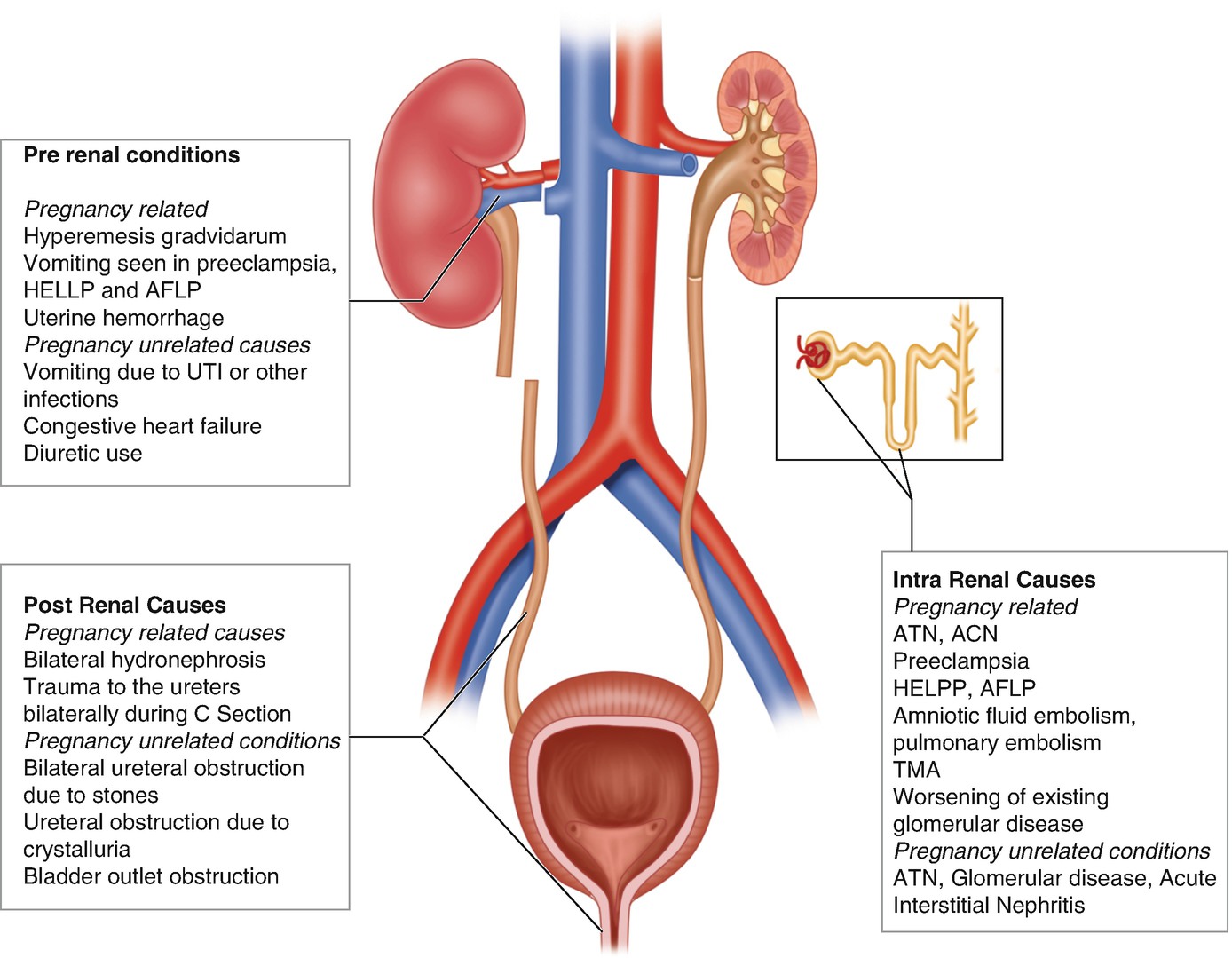
Common causes of AKI during pregnancy
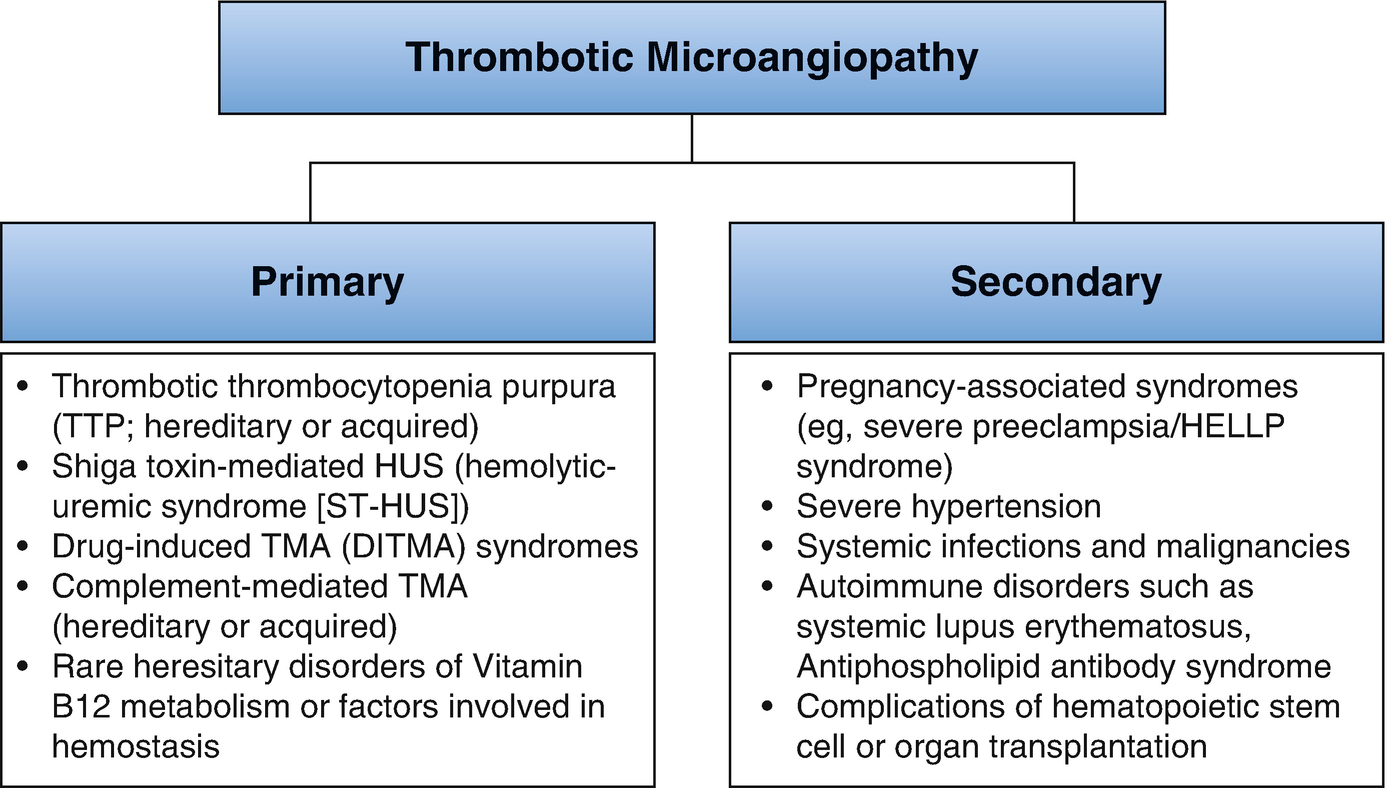
Thrombotic microangiopathy classification. UA/Cr ratio urine albumin to creatinine ratio, U Pr/Cr urine protein to creatinine ratio, FANA fluorescent antinuclear antibody, ANCA antineutrophil cytoplasmic antibody, CT computerized tomography
AKI due to the following pregnancy-specific conditions will be discussed further below: (1) preeclampsia; (2) hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; (3) acute fatty liver of pregnancy (AFLP); (4) thrombotic microangiopathies (TMA); and (5) acute cortical necrosis (ACN). In addition, glomerular diseases can occur de novo or be exacerbated by pregnancy.
Preeclampsia hypertension is commonly seen in modern obstetric practice in part due to an increase in maternal age and with an increase in associated comorbidities such as diabetes mellitus and obesity. Preeclampsia is defined by the development of hypertension (BP greater than or equal to 140/90 at least 4 h apart) in a previously normotensive woman with either proteinuria or end-organ dysfunction typically occurring after 20 weeks of gestation. In preeclampsia there is widespread underlying endothelial dysfunction with inflammation, vasoconstriction, and platelet activation. Eclampsia is the development of new-onset, generalized, tonic-clonic seizures or coma in a woman with preeclampsia. It is sometimes difficult to differentiate preeclampsia from primary glomerular disease especially when prior history is not known. It is also possible to have concomitant disease processes with superimposed preeclampsia in women with prior kidney disease. Fortunately, the risk of developing AKI is only 1% in those with preeclampsia.
Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome is considered by many to be a severe form of preeclampsia and may be seen in 10–20% of women with preeclampsia. AKI in preeclampsia or eclampsia is rarely seen in high-income countries. However it can occur in 3–15% of cases with associated HELLP syndrome and therefore is a leading cause of PR-AKI, accounting for 40–60% of all cases [17–19]. However, AKI in HELLP syndrome, even in the severe forms requiring dialysis, ultimately has a favorable renal outcome. Progression is seen in those with preexisting hypertension and/or renal disease and accounts for less than 10% of patients [18, 20, 21].
Acute fatty liver of pregnancy (AFLP) is a rare condition that occurs in the third trimester of pregnancy and can be associated with preeclampsia in over one half of women, resulting in AKI in some of these patients. The presence of hypoglycemia or encephalopathy and abnormalities in coagulation studies support a diagnosis of AFLP and aid in differentiating it from HELLP. It may recur in following pregnancies, especially if associated with long-chain 3-hydroxyacyl CoA dehydrogenase (LCHAD) deficiency mutations [22, 23].
Acute cortical necrosis (ACN) is rare in developed countries. It is responsible for significant number of cases of AKI in parts of the world where deliveries remote from large cities are common and proper obstetric care is unavailable. Catastrophic obstetric emergencies such as placental abruption with massive hemorrhage, amniotic fluid embolism, DIC, or any condition leading to severe renal ischemia are common predisposing factors. Oliguria or anuria following the inciting event is common presentation. Renal imaging illustrates hypoechoic areas on ultrasound or hypodense areas on CT scan. Renal recovery depends on the extent of parenchyma involved.
Glomerular disease may be preexisting or develop de novo during pregnancy. There may be an acute worsening of preexisting glomerular disease with worsening renal function during pregnancy leading to poor maternal and fetal outcomes. Proteinuria often worsens and hypertension and preeclampsia may develop. Most experts agree that the etiology of glomerular disease (other than lupus nephritis) is probably not a major determinant of worsening renal disease, if factored for preexistent renal insufficiency and hypertension [24, 25]. Lupus nephritis flares during pregnancy can present with increasing proteinuria, hypertension, thrombocytopenia, and a deterioration in renal function. Active lupus nephritis and preeclampsia can also occur at the same time, and distinguishing the two can be difficult. Evidence of lupus activity in other organs can sometimes help distinguish SLE from preeclampsia. Women with lupus are encouraged to delay pregnancy for at least 6 months after the lupus is quiescent for best outcomes. A preconception assessment is essential for risk assessment and to optimize medication regimens with those that have the best safety profile in pregnancy. Presence of antiphospholipid antibodies (aPL) may predispose women to preeclampsia and thromboembolic events. Low-dose aspirin therapy is recommended to minimize risk of complications.
There are many causes of glomerular disease, but the urine sediment and degree of proteinuria help divide them into either nephrotic or nephritic patterns. A spectrum of disease ranging from asymptomatic proteinuria to full-blown nephrotic syndrome with marked edema may be seen. In some cases there is accompanying acute kidney injury, more common with the nephritic glomerular lesions. The etiologies and workup is similar to that in the nonpregnant population. In women with apparent glomerular disease, the urinalysis, the estimated glomerular filtration rate, the degree of proteinuria, and patient characteristics such as age, race, presence of family history of renal disease, and serologic testing help to identify the most likely causes. Systemic manifestations or isolated renal involvement alone may be seen, as may occur in disorders such as systemic lupus erythematosus and vasculitis. Though establishing the correct diagnosis usually requires a renal biopsy, most clinicians have a conservative approach to renal biopsy during pregnancy, and biopsy after 32 weeks is not usually performed.
Thrombotic microangiopathy (TMA) has been reclassified [26] mainly into complement dysregulation TMA, ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 motif 13 repeats)-deficient TMA, and TMA linked to other mechanisms (verotoxin and VEGF deficiency). The etiology and pathogenesis of pregnancy-related TMA (P-TMA) are similar to those in the nonpregnant state with the timing of presentation varying with the type of TMA. There is often an overlap between these conditions. The diagnosis of TMA in pregnancy requires urgent treatment directed at the specific underlying pathophysiology.
ADAMTS13 deficiency-related P-TMA occurs mainly during the second and third trimesters of pregnancy [27]. There is a progressive decline in ADAMTS13 level and an increase in von Willebrand factor (vWF) antigen during normal pregnancy [28, 29] with ADAMTS13 activity to vWF antigen ratio reaching a nadir during the second and third trimesters. This potentiates the inhibitory effect of anti-ADAMTS13 autoantibodies, leading to TMA.
P-TMA caused by complement pathway dysregulation occurs mainly (in 80% of cases) during the postpartum period. Predisposing conditions such as infections and bleeding, occurring in the postpartum period, may trigger complement activation leading to TMA.
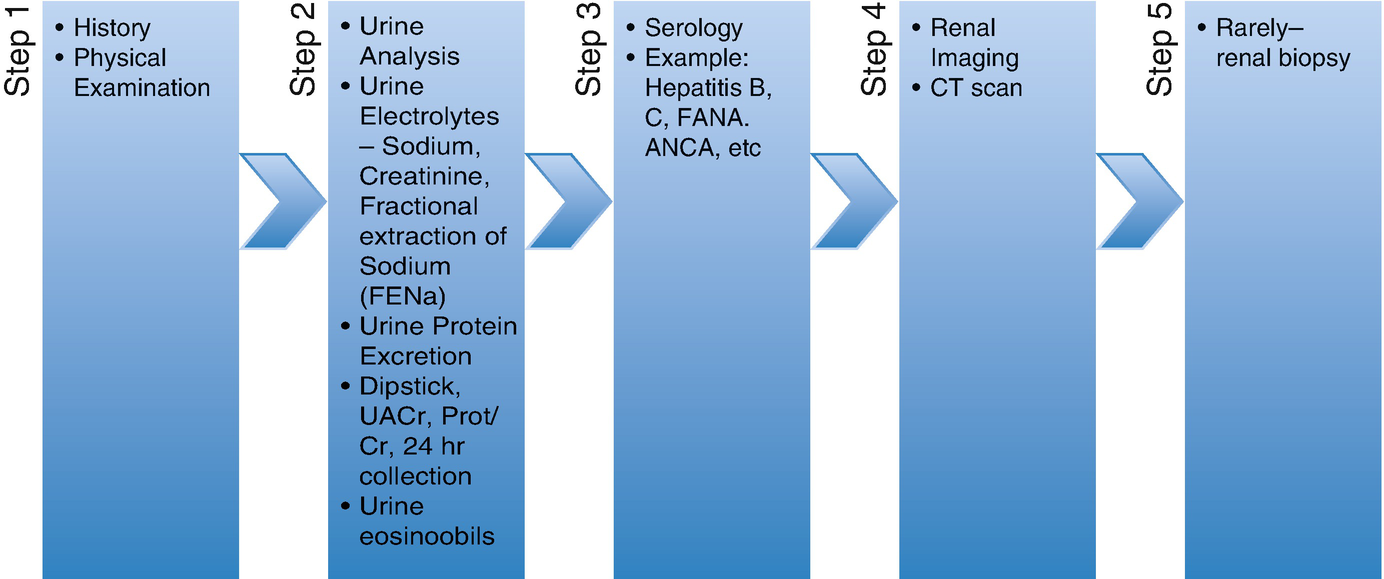
Workup of PR-AKI
Differential diagnosis of AKI with TMA during pregnancy
Disease manifestations and management | Severe preeclampsia/HELLP | AFLP | TTP/HUS | SLE/APLS |
|---|---|---|---|---|
Timing of onset | ||||
Second trimester | + | + | ++ | + |
Third trimester | ++ | ++ | + | + |
Postpartum | + | − | + | + |
Signs and symptoms | ||||
Fever | − | − | + | + |
HTN | +++ | ++ | + | ++ |
Neurological symptoms | + | + | ++ | + |
Purpura | − | − | ++ | + |
Laboratory abnormalities | ||||
AKI | + | ++ | +++ | ++ |
Hemolytic anemia | ++ | + | +++ | ++ |
Thrombocytompenia | ++ | + | +++ | + |
Transaminitis | ++ | +++ | + | − |
DIC | + | ++ | − | − |
Elevated PT | ++ | +++ | − | − |
Hypoglycemia | − | ++ | − | − |
ADAMTS13 deficiency | + | − | ++ | − |
Treatment | ||||
Delivery/supportive | +++ | +++ | − | − |
Plasmapheresis | − | − | +++ | + |
Steroids | +a | +a | +/− | +++ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


