KEY POINTS
• Rh isoimmunization is the primary cause of hemolytic disease of the newborn, although other “irregular” antigens can also be causative.
• All pregnant women must be screened for the presence of circulating antibodies that can cause fetal hemolysis and neonatal hemolytic disease.
• Pregnancies complicated by isoimmunization require assessment by specialists with the clinical experience and ultrasound skills necessary to manage the disease.
• For women who are Rh negative and not sensitized, Rh immunoglobulin can prevent isoimmunization in most situations.
BACKGROUND
Definition
• Isoimmunization, or maternal blood group immunization, is the development of circulating antibodies by the mother directed against an antigen of fetal origin. Isoimmunization is also referred to as maternal sensitization.
• Isoimmunization in pregnancy primarily involves the Rh system of red blood cell antigens, but it can also involve other, “irregular” antigens.
• It is a disease of genetic predisposition, as these antigens are inherited.
• The identification of the rhesus antigen and its relation to hemolytic disease of the newborn was described more than 60 years ago by Landsteiner and Weiner (1) and Levine et al. (2). Their classic works are at the cornerstone of modern immunohematology.
• The evolution of our understanding of the complexities of the Rh blood group system, combined with the development of sensitive screening tests for blood group antibodies, has given us the ability to diagnose and treat this unique disease of pregnancy. Indeed, we now have the capability to both treat and prevent this historically difficult problem.
Pathophysiology
• The pathophysiology of isoimmunization involves the following sequence:
• Maternal sensitization against a fetal blood group antigen
• Maternal production of IgG antibodies
• Transplacental antibody passage to the fetus
• Antibody-mediated hemolysis in the fetal circulation, resulting in fetal anemia
D Antigen
• In the Fischer-Race nomenclature system, the Rh complex contains three pairs of antigenic determinants (Dd, Cc, Ee). The d allele has never been immunologically identified (there is no specific antiserum).
• The presence of the D antigen determines that the individual is Rh positive. The Rh-positive individual is homozygous for D (i.e., DD) approximately 45% of the time. This results from having inherited a D-containing set of alleles from both parents. The remainder of Rh-positive individuals will be heterozygous for the D allele (Dd). A homozygous partner of an Rh-negative woman can produce only Rh-positive offspring; a heterozygous partner can produce either Rh-positive or Rh-negative offspring with equal frequency in each pregnancy. Because the d allele cannot be identified, zygosity at the D locus cannot be known with certainty. However, the Cc, Dd, and Ee alleles are closely linked on chromosome 1, and the statistical likelihood of homo- or heterozygosity can be estimated based on the alleles identified at the Cc and Ee loci.
• Rh isoimmunization can occur only in the pregnancy with an Rh-positive fetus, and only that fetus or subsequent Rh-positive fetuses will be affected by the maternally produced Rh antibody.
• Although the allelic combination at the Cc and Ee loci can be used to predict the statistical likelihood of an Rh-positive fetus, recent advances in DNA testing have allowed direct fetal antigen determination for Rh and other blood group antigens from cultured amniocytes or from chorionic villus sampling.
• More recently, evaluation of circulating cell-free DNA in maternal plasma early in pregnancy has been demonstrated to accurately predict fetal RhD antigen status and is now commercially available (3). Accurate identification of fetal antigen status for other antigens still requires DNA testing from cultured amniocytes or chorionic villus sampling.
Atypical Antibodies
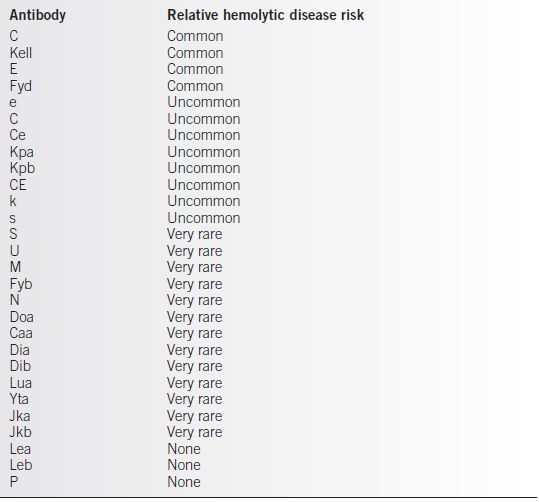
• Antibodies directed against antigens c and E, and less commonly against e and C, are associated with the risk of hemolytic disease and are considered “atypical” antibodies. A number of other red blood cell antigens that are not part of the Rh antigen complex are capable of stimulating isoimmunization as well (Table 35-1) and are also considered atypical antigens. Because of the efficacy with which Rh immunoglobulin prophylaxis programs prevent Rh D isoimmunization, hemolytic disease caused by these atypical antigens has become relatively more important.
• The general principles of management of sensitization with atypical antibodies are similar to those applied in the evaluation and treatment of Rh D isoimmunization. In the past, many have felt that atypical antibody titers did not reliably predict the severity of hemolytic disease; more recent studies suggest that management decisions should be made using the same protocol as for Rh D disease (4). As with all isoimmunized patients, referral to a high-risk center for evaluation and management is prudent.
Epidemiology
• The absence of the Rh antigen (Rh negativity) is predominantly a trait of Caucasians, with 15% to 16% of the Caucasian population identified as Rh negative. However, the distribution is not homogeneous. The Basque population of Spain has an incidence of 30% to 32% Rh negativity.
• The frequency of Rh negativity in Asian, Native American, and African populations is low. Approximately 8% of African Americans are Rh negative.
• These population statistics argue that Rh negativity was originally confined to the Basque population and that initially all of the races were Rh positive.
Etiology
• Blood group isoimmunization generally arises from one of two pathogenic incidents:
• Incompatible blood transfusion
• Fetal–maternal hemorrhage
Incompatible Blood Transfusion
• Transfusion isoimmunization, although uncommon, is generally related to development of atypical blood group antibodies.
• The majority of atypical antibodies are of little clinical consequence; however, severe hemolytic disease of the fetus and newborn can be related to a few atypical antibodies (Table 35-1).
Table 35-1 Atypical Maternal Blood Group Antibodies and Associated Risk of Fetal–Neonatal Hemolytic Disease
Fetal–Maternal Hemorrhage
• Fetal–maternal bleeding is a much more common cause of Rh D isoimmunization than is incompatible blood transfusion, and it is a significant cause of atypical antibody development as well.
• As demonstrated in 1986 by Bowman et al. (5), 75% of pregnancies have some evidence of transplacental hemorrhage during gestation or at delivery. As measured by the sensitive tests developed by Kleihauer et al. (6) in 1957, the amount of fetal blood in the maternal circulation is most frequently less than 0.1 mL.
• Antepartum hemorrhage, gestational hypertension, manual removal of the placenta, cesarean delivery, and external cephalic version are all associated with larger volumes of blood loss and transplacental hemorrhage. Amniocentesis, for genetic or other indications, has also been associated with increased risk of maternal sensitization, particularly if the placenta is traversed during amniocentesis. A 5% to 25% incidence of fetal–maternal hemorrhage has been identified after abortion, both spontaneous and therapeutic.
• Maternal response to the introduction of Rh-positive fetal cells into maternal circulation is a two-step phenomenon.
• The first, primary Rh immune response is frequently slow and weak in its development. This may be related to the relatively compromised immune response seen in the pregnant patient.
 Immunoglobulin M (IgM) anti-D is often not identified before 8 to 9 weeks after exposure, and as many as 6 months may elapse before this primary response is seen.
Immunoglobulin M (IgM) anti-D is often not identified before 8 to 9 weeks after exposure, and as many as 6 months may elapse before this primary response is seen.
 The pregnant patient then frequently switches rapidly to the production of IgG anti-D, which does cross the placenta.
The pregnant patient then frequently switches rapidly to the production of IgG anti-D, which does cross the placenta.
– After the establishment of the primary response, a second exposure with a very small inoculum generally produces a rapid and profound increase in IgG anti-D.
Incidence
• Although the likelihood of Rh immunization appears to be dose dependent, a small inoculum may be sufficient to generate immunization. Zipursky and Israels (7) and Woodrow (8) have reported data indicating that 50% of patients would become immunized with an inoculum of 50 to 75 mL of Rh-positive cells.
• The secondary response, after initial immunization has occurred, can be provoked by as little as 0.1 mL of red blood cells.
ABO Incompatibility
• It appears that the risk of Rh immunization is approximately 16% when an Rh-negative woman has her first pregnancy with an ABO-compatible Rh-positive fetus.
• If the mother is not immunized by the first pregnancy, the risk appears approximately the same for the second pregnancy.
• Although subsequent pregnancy risks for nonresponding patients decrease, a patient undergoing five Rh-positive ABO-compatible pregnancies has a 50% likelihood of becoming Rh immunized.
• An ancillary consideration is the impact of fetal ABO incompatibility, which confers partial protection, thereby decreasing immunization risks markedly. Woodrow (8) calculated that the risk of Rh immunization after an ABO-incompatible Rh-positive pregnancy is approximately 2%.
• Although fetal–maternal hemorrhage sufficient to cause immunization can occur throughout the pregnancy and delivery process, as many as 2% of Rh-negative women become Rh immunized during otherwise uncomplicated pregnancies between 28 weeks of gestation and delivery (9):
• This represents 12% of all Rh-negative women who would become Rh immunized as a result of Rh-positive pregnancies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


