Chapter 449 Iron-Deficiency Anemia
Clinical Manifestations
Iron deficiency has nonhematologic systemic effects. The most concerning effects in infants and adolescents are impaired intellectual and motor functions that can occur early in iron deficiency before anemia develops. There is evidence that these changes might not be completely reversible after treatment with iron, increasing the importance of prevention. Pica, the desire to ingest non-nutritive substances, and pagophagia, the desire to ingest ice, are other systemic symptoms of iron deficiency. The pica can result in the ingestion of lead-containing substances and result in concomitant plumbism (Chapter 702).
Laboratory Findings
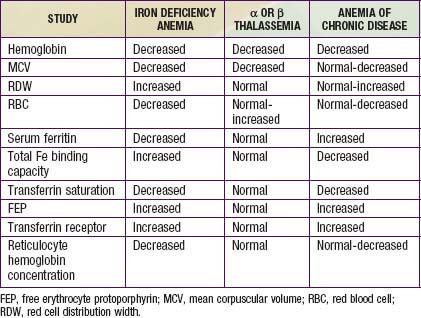
In progressive iron deficiency, a sequence of biochemical and hematologic events occurs (Tables 449-1 and 449-2). Clinically, iron deficiency anemia is not difficult to diagnose. First, tissue iron stores are depleted. This depletion is reflected by reduced serum ferritin, an iron-storage protein, which provides an estimate of body iron stores in the absence of inflammatory disease. Next, serum iron levels decrease, the iron-binding capacity of the serum (serum transferrin) increases, and the transferrin saturation falls below normal. As iron stores decrease, iron becomes unavailable to complex with protoporphyrin to form heme. Free erythrocyte protoporphyrins (FEPs) accumulate, and hemoglobin synthesis is impaired. At this point, iron deficiency progresses to iron-deficiency anemia. With less available hemoglobin in each cell, the red cells become smaller. This morphologic characteristic is best quantified by the decrease in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH). Developmental changes in MCV require the use of age-related standards for diagnosis of microcytosis (see Table 441-1). Increased variation in cell size occurs as normocytic red cells are replaced by microcytic ones; this variation is quantified by an elevated RBC distribution width (RDW). The red cell count (RBC) also decreases. The reticulocyte percentage may be normal or moderately elevated, but absolute reticulocyte counts indicate an insufficient response to the degree of anemia. The blood smear reveals hypochromic, microcytic red cells with substantial variation in cell size. Elliptocytic or cigar-shaped red cells are often seen (Fig. 449-1). Detection of increased transferrin receptor and decreased reticulocyte hemoglobin concentration provides supporting diagnostic information when these studies are available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree