Background
Anecdotal evidence has suggested an association of intravenous drug abuse with alloimmunization; however, published data are limited to case reports.
Objective
The purpose of this study was to determine whether women with a history of intravenous drug abuse have an increased risk of alloimmunization.
Study Design
A retrospective cohort study was performed with the use of data from a single-center blood bank and perinatal database from 2008–2014. Blood bank data were used to identify women with alloimmunization, which was defined as a positive antibody screen in pregnancy not due to naturally occurring antibodies, agglutinins, autoantibodies, or Rh immunoglobulin administration. Intravenous drug abuse was ascertained from a comprehensive database that has captured all drug abuse in pregnancy since 2008. For women who contributed >1 pregnancy to the database, only the most recent pregnancy was included. The rates of alloimmunization among women with a history of intravenous drug abuse and general obstetric populations were calculated and compared. The distribution of alloantibody types, proportion of Rh-group alloantibodies, and patient Rh status were assessed for intravenous and non–intravenous drug abuse–associated alloimmunization. Characteristics and outcomes between intravenous and non–intravenous drug abuse–associated alloimmunization were assessed for women with clinically significant alloantibodies.
Results
Alloimmunization was more common in women with a history of intravenous drug abuse (11/305 women; 3.6%) compared to women without a history of intravenous drug abuse (288/16,022 women; 1.8%; relative risk, 2.00; 95% confidence interval, 1.11–3.62). Needle-sharing was present in 7 and suspected in 4 women with an intravenous drug abuse history. Among women with a history of intravenous drug abuse, none had a history of transfusion or traditional risk factor for alloimmunization. The distribution of alloantibodies was different between intravenous drug abuse– and non–intravenous drug abuse–associated alloimmunization. Rh group alloantibodies and Rh-negative status were more common in women with a history of intravenous drug abuse. Among Rh-negative women with a history of intravenous drug abuse, 50% of RhD alloimmunization cases occurred in nulliparous women. The rate of multiple alloantibodies was not different between intravenous drug abuse– and non–intravenous drug abuse–associated alloimmunization.
Conclusion
Maternal history of intravenous drug abuse is associated with an increased risk of alloimmunization. Approximately 1 in 30 intravenous drug abuse women may be diagnosed with an alloantibody in pregnancy. Given the current US opioid epidemic, increased vigilance in screening is required. Needle-sharing represents a possible mechanism for intravenous drug abuse–associated alloimmunization; however, limited obstetric care, failure to obtain Rh immunoglobulin, or failure to identify early pregnancy loss cannot be excluded.
Alloimmunization typically arises after exposure to foreign red blood cell antigens as the result of pregnancy, transfusion, or transplantation. In pregnancy, fetal-maternal hemorrhage represents the primary exposure that results in alloimmunization through clinical events such as induced or spontaneous abortion, ectopic or molar gestation, invasive procedures such as amniocentesis or chorionic villus sampling, antenatal hemorrhage, placental abruption, or maternal abdominal trauma. Apart from these typical risk factors, injection with needles that are contaminated by foreign red blood cell antigens has been implicated as a risk factor for alloimmunization. Initially reported in 1973, the literature contains a small number of case reports or series on intravenous drug use (IVDU) and needle-sharing that leads to rhesus (Rh) alloimmunization and, in some cases, adverse fetal outcome as the result of hemolytic disease of the fetus and newborn infant (HD). In total, 15 cases have been reported in the literature to date. However, these studies have not used a control group, assessed for potential confounding factors, assessed non-Rh antigens, or characterized the magnitude of the association between IVDU and alloimmunization in pregnancy.
Notably, over the past 2 decades, the diversion and abuse of prescription drugs, particularly opioid pain relievers, has increased markedly in the United States. Between 2002 and 2011, approximately 25 million people initiated nonmedical use of opioid pain relievers, which included many women of reproductive age. Furthermore, the opioid epidemic has resulted in an increase in heroin abuse and heroin-related morbidity and death. Consistent with this trend, observational studies have documented a 5-fold increase in maternal opioid use in pregnancy and a 3-fold increase risk in neonatal abstinence syndrome over the past 15 years.
The routine administration of prophylactic antenatal and postpartum rhesus immune globulin (RhIG) has made Rh alloimmunization a rare encounter in clinical practice in the United States. However, the recent exponential increase in opioid addiction and heroin abuse among women of reproductive age may create a new epidemic of alloimmunization and HD should a link between IVDU and alloimmunization exist. Given the paucity of literature on the association of IVDU and alloimmunization in the context of the current opioid epidemic, our primary objective was to determine the presence and magnitude of the association between IVDU and alloimmunization. We also planned to characterize other pertinent clinical factors of IVDU-related alloimmunization that may be distinct from historic alloimmunization.
Materials and Methods
A retrospective cohort study was performed with data from the blood bank and perinatal database at MetroHealth Medical Center in Cleveland, OH, from 2008-2014. To generate a cohort of women with alloimmunization in pregnancy, which was defined as all women with a red cell alloantibody, institutional blood bank data were used to identify all women of reproductive age (10-50 years old) with alloantibodies on a type and screen during the study epoch. To ensure all significant red blood cell alloantibodies were maintained, the following exclusion criteria were applied to the blood bank dataset: positive antibody screen because of documented RhIG administration, cold and warm agglutinins or autoantibodies, white blood cell antibodies, and samples for which no clinically significant antibody was identified. This group of women with alloantibodies was then merged against the MetroHealth Medical Center perinatal database from the same epoch to generate an alloimmunized cohort of pregnant women. The MetroHealth Medical Center perinatal database contains validated medical, obstetric, and neonatal outcome data for all patients who delivered at the MetroHealth Medical Center since 1974. Obstetric data are entered prospectively into the computerized perinatal database by trained data entry personnel and verified against each patient’s electronic medical record by an independent reviewer. For women who contribute >1 pregnancy to the database, only the most recently pregnancy was included. Finally, for all women with naturally occurring antibodies (such as anti-M or anti-N), charts were reviewed, and women were excluded if chart assessment failed to identify an exposure that could result in sensitization.
In response to the opioid epidemic in Northeast Ohio, MetroHealth Medical Center initiated a Mother and Child Dependency Program in 2008. This program involves a coordinated, multidisciplinary approach to perinatal care that includes maternal fetal medicine, neonatology, nursing, and social work. Detailed social histories are attempted on all patients who are involved in the program that include specific information regarding the type and route of illicit drug use, sexual history, and needle-sharing. An attempt is made to obtain urine toxicology screening at the initial clinical presentation and at delivery on all patients in the program. Since the inception of the program, a separate comprehensive database that captures all substance abuse in pregnancy has been maintained. The substance abuse in pregnancy database served as the source to identify women with the exposure of IVDU in pregnancy. For the purposes of this study, needle-sharing was considered to be present if (1) a specific history was documented in the database (obtained by patient history) or if (2) women had a history of both IVDU and hepatitis C or HIV seroconversion. The latter definition was included given that parenteral exposure is the most effective mechanism of hepatitis C virus (HCV) transmission and that previous epidemiologic evidence has reported that approximately 75% of patients with IVDU in the United States have HCV. Therefore, seroconversion with IVDU most likely represents needle-sharing. Needle-sharing was considered “suspected” among women with a history of IVDU and new alloantibodies if there was not documentation of needle-sharing and serostatus for HIV and HCV was negative. Chart review was performed on all women with IVDU and alloimmunization to validate exposure and substance abuse history.
Based on the underlying pathophysiologic mechanism that IVDU and needle-sharing results in potential exposure to all foreign red cell antigens, we first assessed the overall rates of alloimmunization in the IVDU and general obstetric populations. The distribution of alloantibody types, proportion of Rh-group alloantibodies, and patient Rh status were assessed for IVDU- and non–IVDU-associated alloimmunization. Additional analysis was then performed on the subgroup of women with clinically significant alloantibodies, which was defined as those implicated in HD. For this subgroup analysis, all cases of alloimmunization due to antibodies that are not associated with HD were then excluded. The charts of all patients with clinically significant alloantibodies were abstracted. Detailed maternal data were obtained on pregnancy and obstetric history, risk factors for alloimmunization including invasive fetal testing (chorionic villus sampling, amniocentesis, multifetal pregnancy reduction), history of trauma or transfusion, maternal alloantibody titers, paternal zygosity testing, and antenatal testing and/or fetal transfusion. Abstracted neonatal data included initial hemoglobin and the diagnosis and treatment for HD including neonatal transfusion. Maternal and neonatal characteristics and outcomes between IVDU- and non–IVDU-associated alloimmunization were compared. Categoric variables were analyzed with the use of chi-squared or Fisher’s exact test, where appropriate. Continuous variables were analyzed with the use of unpaired t -tests and Wilcoxon Rank Sum, as appropriate. Analysis was performed with Stata software (version 13.1; Stata Corporation, College Station, TX). Institutional review board approval was obtained before study initiation (IRB#15-00302, approved June 14, 2015).
Results
During the study epoch, after the exclusion of the women who contributed multiple pregnancies to the database and the maintenance of only the most recent pregnancy, 16,327 women delivered at MetroHealth Medical Center of whom 305 women (1.9%) were identified to have a history of IVDU. Blood bank data identified 5251 women of reproductive age with a positive antibody screen from 2008-2014. Application of exclusion criteria and generation of the final cohort of women with alloimmunization in pregnancy is depicted in Figure 1 .
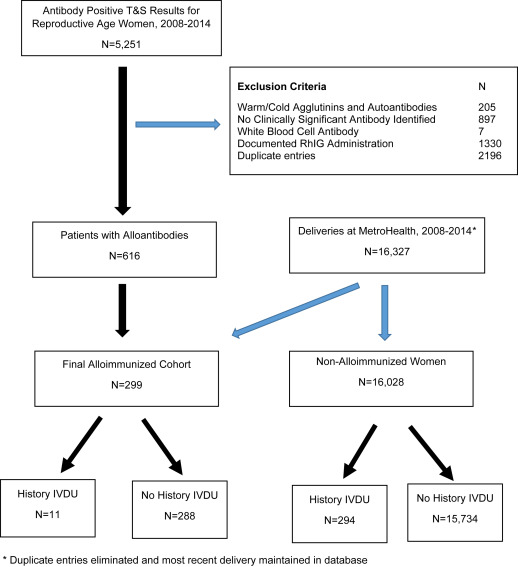
Alloimmunization was more common in women with a history of IVDU (11 of 305, 3.6%) compared to women without a history of IVDU (288 of 16,022, 1.8%; P = .02, relative risk 2.00, 95% confidence interval 1.11-3.62). The distribution of alloantibodies was different between IVDU- and non–IVDU-associated alloimmunization ( Figure 2 ). Antibody characteristics and Rh status for the cohort are outlined in Table 1 . Rh-negative status and Rh-group alloantibodies were more common in women with IVDU. Four Rh-negative women with a history of IVDU had anti-Rh group alloantibodies, all of which were anti-D. However, 50% of these Rh-D alloimmunized women were nulliparous. The rate of multiple alloantibodies was not different between IVDU and non-IVDU alloimmunization (27.2% vs 8.0%; P = .06). Chart review confirmed that needle-sharing was present in 7 and suspected in 4 women with IVDU. Notably, no women with IVDU-associated alloimmunization had a history of transfusion or other common risk factor for alloimmunization (eg, abruption, fetal maternal hemorrhage, invasive procedure in pregnancy). Urine toxicology screening obtained either at initial presentation to care or delivery was obtained on 283 of 305 women (92.8%). Of the women with toxicology screening, results were positive for opiates in 155 (54.8%), for methadone or buprenorphine in 141 (49.8%), for benzodiazepines in 61 (21.6%), for tetrahydrocannabinol in 41 (14.5%), and for cocaine or other drugs in 59 (20.9%).
| Variable | Intravenous drug use (n = 11), n (%) | No intravenous drug use (n = 288), n (%) | P value a |
|---|---|---|---|
| Women with Rh-group alloantibodies | 8 (72.7) | 84 (29.2) | .004 |
| Rh-negative women | 5 (45.5) | 27 (9.4) | .003 |
| Rh-negative women with Rh-group alloantibody b | 4 (80.0) | 14 (51.9) | .34 |
| Women with multiple alloantibodies | 3 (27.2) | 23 (8.0) | .06 |
a Calculation with the use of chi-squared or Fisher’s exact test, as appropriate
b All 4 Rh-negative women with Rh-group alloantibodies and a history of intravenous drug use had anti-D antibodies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


