Intrauterine Growth Restriction
Joseph B. Warshaw
Intrauterine growth restriction (IUGR), formerly referred to as intrauterine growth retardation, represents a final common pathway by which genetic and environmental influences result in low birth weight for gestational age. IUGR has been defined most commonly in the United States as a birth weight of less than the tenth percentile for gestational age. This definition probably overestimates the incidence of IUGR, because it is unreasonable to consider that 10% of all births have a pathologic restriction of growth. Small infants with no evidence of adverse genetic or environmental influences should be spared the IUGR label, which connotes pathology, and should be defined as small for gestational age (SGA). The term SGA should be applied to all infants less than the tenth percentile, and IUGR generally should be reserved for infants less than the third percentile, recognizing that some infants with growth restriction will fall out of this range if an insult occurs late in gestation. Thus, although all infants with IUGR also are SGA, not all SGA infants have IUGR.
Confusion about definitions is increased further by significant differences in the tenth percentile birth weights at each gestational age that have been published. Differences in published standards of growth have been influenced by racial composition, socioeconomic status of the population, and altitude above sea level when the standards were developed. What is necessary for an effective comparison between populations is the adoption of a single standard for fetal growth (e.g., the standards developed by Brenner from 30,772 deliveries from 21 to 44 weeks’ gestational age in Cleveland). The Brenner standards include correction factors for poverty, race, and gender.
ETIOLOGY OF IUGR
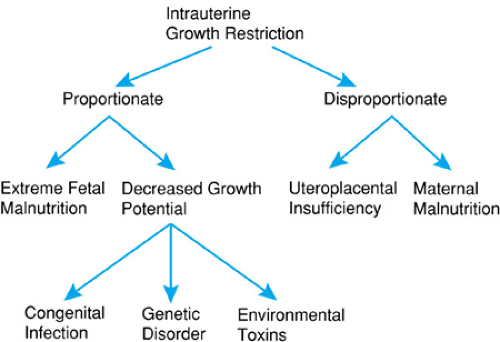
Recognition and treatment of IUGR require an understanding of the diverse etiologies that result in restricted fetal growth. The growth pattern of the infant with IUGR often reflects the underlying condition that has resulted in growth restriction. The terms proportionate and disproportionate have been used to distinguish newborns with decreased growth potential from those with restricted growth because of fetal malnutrition. There may be intergenerational risks for IUGR. Mothers who were SGA at birth have twice the risk of having an infant with IUGR.
Newborns with decreased growth potential caused by conditions such as chromosomal disorders, congenital infections, or exposure to environmental toxins characteristically have body proportions that are proportionate or symmetric (i.e., the head, length, and weight generally occur within similar percentile grids), or the head is small relative to the body, as in microcephaly. Obstetric monitoring of the fetus with decreased growth potential characteristically demonstrates decreased body growth, including that of the head, from midgestation or earlier. Fetuses with decreased growth potential are at high risk for major malformations or congenital infection.
Newborns with fetal malnutrition have reduced weight out of proportion to length or head circumference and may exhibit a sparing of head growth during late gestation. These infants are disproportionate, with the head circumference and length closer to the expected percentiles for gestational age than those for weight. Nutritional constraints on growth are unusual before 24 to 25 weeks’ gestation except in a multiple pregnancy. In most cases, only after that time will restriction in blood supply to the fetus result in IUGR. In mild to moderate degrees of IUGR, head growth may proceed along normal percentile grids, with a decrease in body fat and restriction in length and weight (disproportionate growth). This sparing of head growth is thought to result from circulatory changes in the fetus that favor a redistribution of blood flow to the heart and brain. Doppler blood flow measurements of the fetus have shown increased carotid and decreased visceral blood flow in fetuses with IUGR as compared to those with normal growth. These changes may be mediated by release of vasopressin from the fetal hypothalamus or by prostaglandins. Exceptions to this general pattern may be seen. In instances of extreme nutritional restriction in the fetus with class D diabetes or other conditions that result in severely compromised uterine blood flow, even head growth may be decreased.
Infants with either proportionate or disproportionate IUGR should be evaluated carefully for conditions causing hydrocephalus or microcephaly that also may confound the measurements. Figure 27.1 summarizes the etiology of IUGR. The importance of environmental exposures such as cigarette smoking cannot be overestimated. In the developed countries of the world, cigarette smoking remains an important determinant of low birth weight. Cigarette smoking among women in the developing world is increasing, and remains a concern about teenagers in the United States.
The pattern of postnatal growth is also important to record and follow. As a consequence of decreased growth potential, infants with proportionate growth restriction may exhibit sluggish postnatal growth even with adequate nutrition. A slow rate of postnatal growth may be seen in genetic disorders, congenital infections, or fetal alcohol syndrome. Infants with growth restriction secondary to fetal malnutrition often exhibit rapid growth when adequate nutrients are provided in the postnatal period, which is a good prognostic finding. Approximately 30% of nutritionally induced newborns with IUGR are still below the third percentile at 2 years of age.
MANAGEMENT
Optimal management of IUGR should begin with recognition of the problem in utero so that informed decisions can be made concerning the appropriate time and method of delivery. This includes consideration of the options of cesarean section versus vaginal delivery. If biophysical data obtained during fetal monitoring show fetal distress, cesarean section may be the preferred mode of delivery. Decreased maternal weight gain or very low prepregnancy weight should alert the obstetrician to the likelihood of fetal growth restriction. Measurement of fundal growth and ultrasonography can confirm the diagnosis by monitoring such parameters of fetal growth as the biparietal diameter, the relationship of head size to body size, femur length, and fetal abdominal circumference.
Strategies to treat fetal growth restriction have included therapies to decrease platelet aggregation and abnormalities in uteroplacental circulation seen in toxemia of pregnancy as well as maternal nutritional supplementation and oxygen therapy. These potential therapies require further investigation.
Maternal parenteral nutritional supplementation is controversial. An adverse influence was observed when short-term administration of glucose to normal patients before delivery resulted in a significant increase in lactic acid and a decrease in pH. When the fetus is adapted to a decreased nutrient supply, a potential risk may be associated with increasing nutritional intake without a corresponding increase in fetal oxygenation. Indeed, several of the changes seen in IUGR can be considered adaptations to an adverse intrauterine nutritional environment: sparing of brain growth, increased red cell mass, and small size itself, in which the size of the fetus may be appropriate to the availability of nutrients. Even early lung maturation can be considered an adaptation that improves the opportunity for a good postnatal outcome. There is also some evidence that the calorically restricted fetus can regulate its own growth. In animal studies, nutritionally deprived fetuses have decreased levels of insulin-like growth factors (IGF) and an increased level of IGF binding protein. This effectively modulates fetal growth by decreasing the growth-promoting effect of the IGF. A smaller fetus with IUGR would have decreased caloric requirements, which can be advantageous for the fetus. Similar data have been observed in stressed newborns.
Important problems of the infant with IUGR secondary to intrauterine malnutrition are summarized in Box 27.1. Appropriate management can prevent many of these problems (Box 27.2). If birth asphyxia occurs, support measures should be instituted immediately, including the establishment of an effective airway and the management of meconium if present. Suctioning and clearing the meconium from the airway before delivery of the thorax and the infant taking the first breath, together with tracheal suction if meconium is present at the level of the cords, have greatly reduced problems associated with meconium aspiration. Meconium aspiration is rarely seen in infants of less than 35 weeks’ gestation; therefore, hypoxia per se is the major problem in those infants. An estimate of the degree of acidosis can be obtained from the cord blood pH. Hyaline membrane disease is generally less of a problem in infants with disproportionate IUGR because of the accelerated lung maturation commonly seen in these infants. Some asphyxiated newborns exhibit significant right-to-left cardiac shunting, making systemic oxygenation difficult to achieve. This is a consequence of chronic intrauterine hypoxia, which results in abnormal thickening of the smooth muscle of small pulmonary arterioles, thereby reducing pulmonary blood flow and increasing right-to-left blood flow at the atrial level or through the ductus arteriosus. Diagnosis is suggested by pulse oximetry and in most cases can be confirmed by Doppler flow measurements of right-to-left shunting. Diagnosis can also be confirmed by measuring the disparity between preductal (right radial) and postductal (umbilical arterial) PO2. Because acidosis and hypoxia modulate pulmonary arteriolar vasomotor tone, therapeutic efforts should be directed toward reversing these conditions. Some infants appear to improve with an induced respiratory alkalosis (pH 7.45 to 7.55). Treatment of persistent pulmonary hypertension has been revolutionized by nitric oxide therapy, which is effective in reversing pulmonary hypertension in most cases. Extracorporeal membrane oxygenation had a surge in popularity in the treatment of this condition, but the efficacy of this expensive and complex intervention has not been clearly established and has, in most cases, been superceded by nitric oxide therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree