Fig. 5.1
Arteriovenous malformation (AVM): a Pulsatile mass of the right cheek. b Arterial and c venous phases of right external carotid angiogram demonstrating high-flow AVM with large draining veins. d Unsubtracted lateral fluoroscopic image demonstrating opacified Onyx embolic agent within the feeding vessels. e Ultrasound image of percutaneous needle access of abnormal vein. f Fluoroscopic image with subtraction of contrast injection of the abnormal vein
MRI provides the best spatial resolution for soft tissue, with computed tomography (CT) better delineating any osseous abnormality, when the lesion is in close association with the bone. Catheter angiography remains the gold standard in terms of providing the highest spatial resolution, as well as critical insight into the flow dynamics (Fig. 5.1b, c), though catheter-based procedures are usually performed as part of the therapeutic approach, rather than purely for diagnosis. High-flow vascular malformations are usually complex lesions where the therapeutic goal is symptom control, preservation of vital functions (e.g., vision, hearing, or mastication), or aesthetic restoration, rather than cure, although for focal lesions, a combination of single or multistage preoperative embolization followed by surgical resection can sometimes be curative [1, 3, 4].
Endovascular embolization is directed towards occlusion of the nidus and initial segment of the venous outflow. This can either be performed transarterially or by percutaneous direct access of the nidus or the draining vein. Preoperative embolization provides a dry surgical field and minimizes perioperative blood loss. Gelfoam powder, polyvinyl particles, or embospheres can be used for temporary preoperative embolization. For nonoperative candidates, embolization with permanent liquid agents capable of permeating the AVM nidus, such as absolute ethanol, n-Butyl Cyano Acrylate (glue), or Onyx, may be used (Fig. 5.1d–f) .
Endovascular embolization can be highly effective in cases of arterio-venous fistulas, both for preoperative adjunctive treatment and as a stand-alone cure. In contrast, for focal AVMs with multiple, small feeders, nidal embolization followed by surgical resection is the usual treatment [5].
Low-Flow Malformations
These lesions are broadly classified as capillary, venous, or lymphatic malformations .
Capillary Malformations (CM)
These are flat, well demarcated, lesions showing ectatic blood vessels in the dermis associated with reduction in innervation, occurring most commonly in the trigeminal V1 distribution [6, 7]. They can be seen in association with syndromes like Sturge–Weber syndrome, Klippel–Trenaunay syndrome, Parkes Weber syndrome, macrocephaly-capillary malformation syndrome, and capillary malformation-arteriovenous malformation syndrome (CM-AVM). The standard treatment is pulsed-dye laser, although only 15–20 % of lesions clear completely [8].
Venous Malformations (VMs)
These represent congenital anomalies, irregular endothelial-lined channels, with thin walls deficient in smooth muscle. They typically have a bluish purplish hue, and are soft and compressible. 40 % of these lesions are found in head, neck, and extremities [9]. Episodic focal thrombosis and occurrence of phleboliths may result in swelling and pain. Larger lesions on the face can cause facial asymmetry. Trauma or hormonal changes can induce enlargement of VM, and they can extend deeper intrafascially and cause mass effect in small anatomical spaces like the orbit and oral cavity. Syndromes like glomuvenous malformation, cutaneomucosal venous malformation, and blue rubber bleb nevus syndrome have VMs as part of their manifestation [10, 11]. VMs most characteristically enhance avidly but in a patchy, heterogeneous pattern on contrast enhanced MRI. Phleboliths are typically seen as hypointense defects on MRI or as calcified foci on CT scan images (Fig. 5.2d) [12].
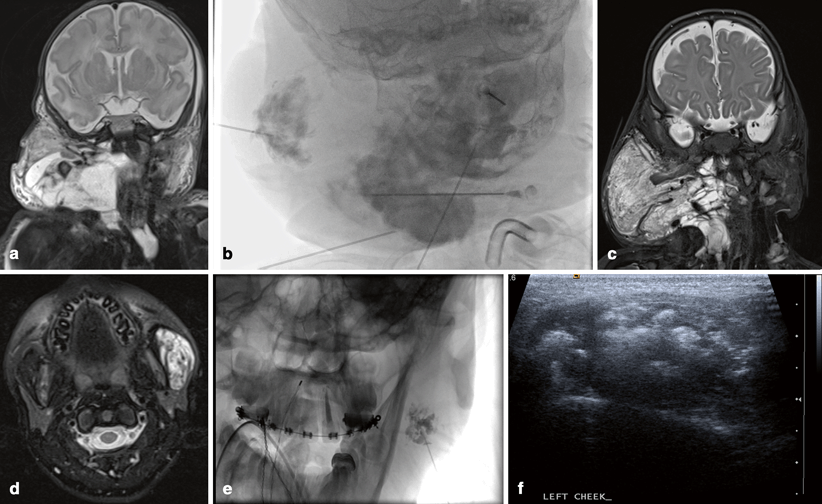
Fig. 5.2
Low-flow lesions: a Coronal T2-weighted MRI of newborn with large multicystic cervicofacial mass, consistent with macrocystic lymphatic malformation. b Anteroposterior (AP) fluoroscopic image of the neck during percutaneous sclerotherapy of multiple macrocysts. c Postsclerotherapy MRI demonstrating reduction of the macrocystic component and persistent microcystic disease. d Axial inversion-recovery MRI demonstrating VM of the left cheek. e AP fluoroscopic image during percutaneous sclerotherapy of the VM. f Ultrasound imaging of the VM after sclerotherapy injection. The echogenicity is secondary to air from foaming of the sclerosing agent
Lymphatic Malformations (LM)
These can be either macrocystic, microcystic, or of combined types. They are soft, noncompressible, translucent masses with overlying normal or bluish skin, often with superficial dry or weeping cutaneous vesicles. Macrocystic LMs have a predilection for the head and neck region. Sudden enlargement following infection or intralesional hemorrhage and spontaneous involution are common. Syndromes associated with LM include Klippel–Trenaunay, Turner, Noonan, and trisomies 13 and 18, and others [9]. On imaging, LMs show variably sized cysts in the macrocystic type, showing debris within or fluid–fluid levels with heterogeneous signal, due to repeated hemorrhages (Fig. 5.2a). Only the septae of macrocysts enhance. Microcystic disease on ultrasound is seen as an echogenic ill-defined mass with tiny, poorly visible cysts. On contrast MRI, microcystic lesions may or may not enhance, with the likelihood of enhancement increased in the setting of inflammation or infection.
Treatment of Low-Flow Malformations: Percutaneous Sclerotherapy
Most interventional-radiology- guided therapy of low-flow vascular malformations involves percutaneous sclerotherapy. Sclerotherapy is injection of a pharmacological agent that induces endothelial damage, elicits an avid inflammatory response, and finally leads to thrombosis (in VMs) and fibrosis. Image guidance, especially ultrasound in children, is most commonly used to gain access into the abnormal vascular channels. Digital subtraction angiograms using fluoroscopy prior to the actual injection of the sclerosant is performed to evaluate the position of the needle tip, the communications between the different components of the malformation, and the local vascular anatomy, including the hemodynamics of the venous drainage. Fluoroscopy can also help with estimation of the volume of sclerosant needed .
The sclerosant is usually reconstituted with a contrast agent, either water soluble, lipophilic (such as ethiodol), or negative contrast (air or carbon dioxide) to allow fluoroscopic and ultrasound monitoring of the injection [12] (Fig. 5.2b, e, f). Vigilantly watching for any extravasations during injection is mandatory, to prevent tissue or nerve damage. Applying direct pressure over venous drainage pathways during injection, using a tourniquet, or using double needle technique, which provides a low-pressure exit valve, can stop drainage to critical outflow veins. Escape of sclerosant into the venous drainage could potentially result in ophthalmic, cavernous, or intracranial venous thrombosis in head and neck lesions .
We most commonly use 3 % sodium tetradecyl sulfate (STS), a detergent, as the sclerosant Foaming the solution prior to injection has been reported to increase efficacy, perhaps by maximizing the surface area contact between the agent and the lesional endothelium. Ethanol, the most potent sclerosant, and one we make regular but judicious use of as well, unfortunately, has the highest rate of reported serious complications such as skin necrosis, nerve damage, central nervous system depression, acute pulmonary hypertension, thromboembolism, disseminated intravascular coagulation (DIC), hyperthermia, cardiac arrhythmias, and cardiovascular collapse and death [13]. However, STS and related detergents can cause serious adverse effects as well. Platinum coils or liquid embolics may serve as adjuncts to sclerotherapy in larger lesions, primarily to close prominent or recurrent venous channels. These agents are particularly effective in achieving preoperative short-term occlusion [12]. Bleomycin, an antibiotic with cytotoxic properties, can be of particular use in patients with intra-orbital and airway lesions, because of significantly less posttreatment edema than is seen with other agents [14]. Presclerotherapy steroids are imperative in orbital and airway malformations, for reducing postprocedural edema, which could result in increased intraocular pressure or airway compression. Sclerosants cause immediate local hemolysis and subsequent hemoglobinuria, though lesions in the head and neck are rarely large enough for the hemolysis to cause systemic problems. Generous hydration (doubling of the maintenance intravenous fluid for 4 h post procedure), monitoring of urinary output, and urinary alkalization with sodium bicarbonate intravenous fluid is recommended [12] .
Localized VMs have the best responses to sclerotherapy. Diffuse malformations are less likely to have a complete response, and the treatment should, therefore, be targeted at the most symptomatic portions. For all but the smallest lesions, sclerotherapy is often repeated. Among the LMs, the macroystic variety typically responds well to sclerotherapy, whereas microcystic lesions are technically difficult to treat and show a poor response (Fig. 5.2c). Sclerosants reported for use in treating macrocystic LMs include ethanol, doxycycline, bleomycin, Ethibloc, and OK-432. Our first-line agent is most commonly doxycycline at a concentration of 10 mg/ml. For large cysts, a pigtail catheter aspiration of the contents and volume measurement is made, followed by injection and drainage of the cyst with the sclerosant. The sclerosant is allowed to dwell in the cyst for 2–3 h and then drained out. The procedure is repeated sequentially on days 2 and 3, through the indwelling catheters. It is important to disrupt the internal septations to increase the contact of the sclerosants within different compartments. Cyst involution can be assessed approximately 6 weeks after the procedure. For microcystic LM, sclerotherapy using bleomycin or OK-432 is often used. Other techniques using in-column electrocoagulation, carbon dioxide laser excision and radiofrequency ablation (RFA) have also been described [14–16]. The overall complication rate for sclerotherapy to treat VMs is 12 % [13]. Peripheral neuropathy is seen in approximately 1 %, but can be avoided if care is taken not to cause extravasation during injection; when it occurs, neuropathy is usually transient. Skin blistering and, in rare occurences, skin necrosis with permanent scarring can occur, particularly when the lesion has a more superficial component. For lesions involving the tongue, buccal surfaces, soft palate, or airway, marked postprocedural edema can cause transient dysphagia and breathing difficulties. Many such patients have a tracheostomy placed before commencing the procedure. Other lesser adverse effects include muscle atrophy and contracture if the sclerosant infiltrates the tissues [12] .
Juvenile Naso-Pharyngeal Angiofibroma (JNA)
JNA is a benign vascular tumor composed of a rich vascular network within a fibrous stroma [17]. It most commonly arises in the posterolateral nasopharynx of prepubertal and adolescent males (Fig. 5.3a). JNA can behave aggressively and tend to bleed frequently. They can expand commonly beyond the nasopharynx into the cranium, nose, and paranasal sinuses [17, 18]. Profuse intraoperative bleeding leading to incomplete resection and tumor recurrence can occur, and preoperatively transarterial tumor embolization can greatly facilitate resection. JNAs are primarily fed from distal internal maxillary artery branches (Fig. 5.3b), and may recruit arterial supply from any nearby ipsilateral or contralateral vessel, requiring bilateral internal and external carotid arteriography for elucidation. Anastomosis between branches of the external and internal carotid artery and vascular spasm has to be considered when planning superselective embolization. Silastic spheres, Gelfoam, dura mater, and polyvinyl alcohol (PVA) particles (Fig. 5.3c) have been used to embolize the tumor bed and the feeding vessels [19], with PVA particles often preferred, as they are efficient and cost effective. Nontarget embolization of particles to the ophthalmic artery, the internal carotid, or vertebral arteries via anastomosis or reflux of particles injected in the external carotid artery may cause retinal or brain ischemic deficits, and thus preembolization and intraprocedural angiography must be scrupulously studied .
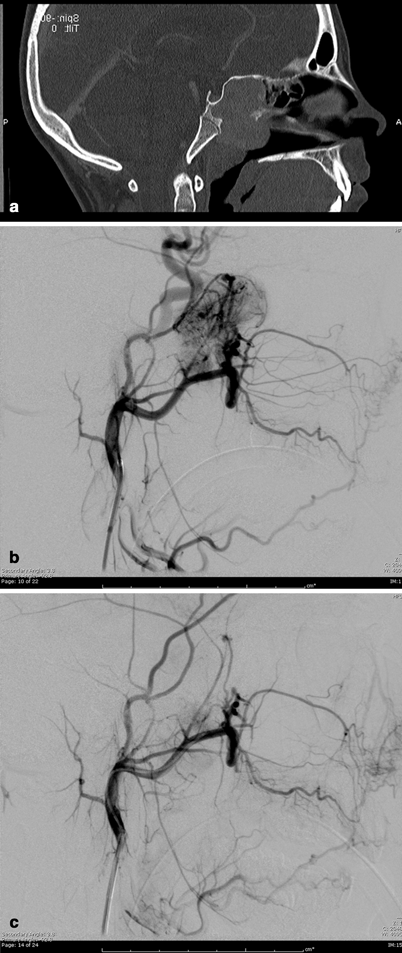
Fig. 5.3
Juvenile nasopharyngeal angiofibroma (JNA): a Sagittal CT-reconstructed image of the head demonstrates large enhancing nasopharyngeal mass. b Selective internal maxillary artery injection demonstrating hypervascular nasopharyngeal mass. c After selective embolization with PVA particles, there is near-complete cessation of flow to the mass with preservation of the normal circulation
Glomus Tumors
Paragangliomas, also called glomus tumors, are highly vascularized tumors of neural crest origin that are derived from chemoreceptor organs in the walls of blood vessels or specific nerves in the head and neck area. They can develop in the middle ear (glomus tympanicum), the jugular foramen of the skull base (glomus jugulare), or other head and neck areas (glomus caroticum, glomus vagale). They are usually benign but locally destructive [20–23]. Preoperative embolization for devascularization greatly reduces the perioperative blood loss by a factor of 2–3, with reduction of need of transfusion in the postoperative period to less than 50 % [24]. However, extreme caution is warranted during embolization of these lesions. In particular, during embolization of carotid body tumors, particles can escape into the internal carotid artery and result in stroke, especially if particles < 150 μm in size are used. As in the case of JNA, vigilant angiographic search for anastomosis between the intra- and extracranial circulation is imperative. Collaterals between the vertebral artery and the C1, C2, and C3 musculoskeletal branches are common. Lower cranial nerves, such as the facial nerve or hypoglossal nerve can undergo ischemia if the vaso nervorum is inadvertently embolized. When embolizing glomus tumors, preprocedural administration of an alfa-blocking agent is often necessary to reduce catecholaminergic activity. Not infrequently, complete arterial devascularization of the tumor bed is not achieved, and several groups have recently described direct puncture and the slow injection of acrylic glue to allow for permeation of the vascular tumor bed while avoiding its passage to the venous side or its reflux into normal arterial territory [25] .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


