Chapter 328 Inflammatory Bowel Disease
It is usually possible to distinguish between ulcerative colitis and Crohn disease by the clinical presentation and radiologic, endoscopic, and histopathologic findings (Table 328-1). It is not possible to make a definitive diagnosis in ∼10% of patients with chronic colitis; this disorder is called indeterminate colitis. Occasionally, a child initially believed to have ulcerative colitis on the basis of clinical findings is subsequently found to have Crohn colitis. This is particularly true for the youngest patients, because Crohn disease in this patient population can more often manifest as exclusively colonic inflammation, mimicking ulcerative colitis. The medical treatments of Crohn disease and ulcerative colitis overlap.
Table 328-1 COMPARISON OF CROHN DISEASE AND ULCERATIVE COLITIS
| FEATURE | CROHN DISEASE | ULCERATIVE COLITIS |
|---|---|---|
| Rectal bleeding | Sometimes | Common |
| Diarrhea, mucus, pus | Variable | Common |
| Abdominal pain | Common | Variable |
| Abdominal mass | Common | Not present |
| Growth failure | Common | Variable |
| Perianal disease | Common | Rare |
| Rectal involvement | Occasional | Universal |
| Pyoderma gangrenosum | Rare | Present |
| Erythema nodosum | Common | Less common |
| Mouth ulceration | Common | Rare |
| Thrombosis | Less common | Present |
| Colonic disease | 50-75% | 100% |
| Ileal disease | Common | None except backwash ileitis |
| Stomach-esophageal disease | More common | Chronic gastritis can be seen |
| Strictures | Common | Rare |
| Fissures | Common | Rare |
| Fistulas | Common | Rare |
| Toxic megacolon | None | Present |
| Sclerosing cholangitis | Less common | Present |
| Risk for cancer | Increased | Greatly increased |
| Discontinuous (skip) lesions | Common | Not present |
| Transmural involvement | Common | Unusual |
| Crypt abscesses | Less common | Common |
| Granulomas | Common | None |
| Linear ulcerations | Uncommon | Common |
Extraintestinal manifestations occur slightly more commonly with Crohn disease than with ulcerative colitis (Table 328-2). Growth retardation is seen in 15-40% of children with Crohn disease at diagnosis. Of the extraintestinal manifestations that occur with IBD, joint, skin, eye, mouth, and hepatobiliary involvement tend to be associated with colitis, whether ulcerative or Crohn. The presence of some manifestations, such as peripheral arthritis, erythema nodosum, and anemia, correlates with activity of the bowel disease. Activity of pyoderma gangrenosum correlates less well with activity of the bowel disease, whereas sclerosing cholangitis, ankylosing spondylitis, and sacroiliitis do not correlate with intestinal disease. Arthritis occurs in 3 patterns: migratory peripheral arthritis involving primarily large joints, ankylosing spondylitis, and sacroiliitis. The peripheral arthritis of IBD tends to be nondestructive. Ankylosing spondylitis begins in the 3rd decade and occurs most commonly in patients with ulcerative colitis who have the human leukocyte antigen B27 phenotype. Symptoms include low back pain and morning stiffness; back, hips, shoulders, and sacroiliac joints are typically affected. Isolated sacroiliitis is usually asymptomatic but is common when a careful search is performed. Among the skin manifestations, erythema nodosum is most common. Patients with erythema nodosum or pyoderma gangrenosum have a high likelihood of having arthritis as well. Glomerulonephritis, uveitis, and a hypercoagulable state are other rare manifestations that occur in childhood. Cerebral thromboembolic disease has been described in children with IBD.
Table 328-2 EXTRAINTESTINAL COMPLICATIONS OF INFLAMMATORY BOWEL DISEASE
MUSCULOSKELETAL
SKIN AND MUCOUS MEMBRANES
DERMATOLOGIC
OCULAR
BRONCHOPULMONARY
CARDIAC
MALNUTRITION
HEMATOLOGIC
RENAL AND GENITOURINARY
PANCREATITIS
HEPATOBILIARY
ENDOCRINE AND METABOLIC
NEUROLOGIC
G6PD, glucose-6-phosphate dehydrogenase; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis.
Modified from Kugathasan S: Diarrhea. In Kliegman RM, Greenbaum LA, Lye PS, editors: Practical strategies in pediatric diagnosis and therapy, ed 2, Philadelphia, 2004, Saunders, p 285.
328.1 Chronic Ulcerative Colitis
Differential Diagnosis
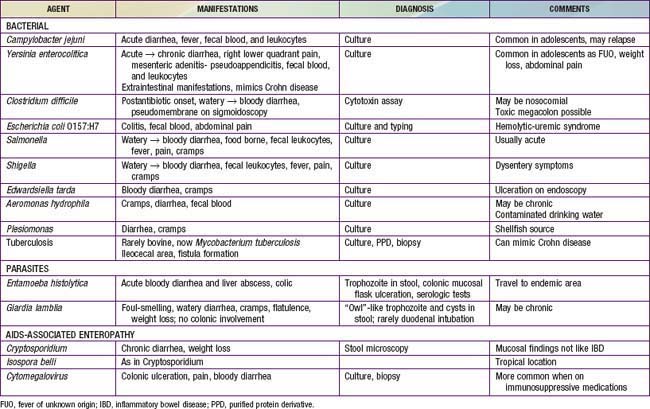
The major conditions to exclude are infectious colitis, allergic colitis, and Crohn colitis. Every child with a new diagnosis of ulcerative colitis should have stool cultured for enteric pathogens, stool evaluation for ova and parasites, and perhaps serologic studies for amebae (Table 328-3). In the setting of antibiotic use, pseudomembranous colitis secondary to Clostridium difficile should be considered. Cytomegalovirus infection can mimic ulcerative colitis or be associated with an exacerbation of existing disease. The most difficult distinction is from Crohn disease because the colitis of Crohn disease can initially appear identical to that of ulcerative colitis, particularly in younger children. The gross appearance of the colitis or development of small bowel disease eventually leads to the correct diagnosis; this can occur years after the initial presentation.
At the onset, the colitis of hemolytic-uremic syndrome may be identical to that of early ulcerative colitis. Ultimately, signs of microangiopathic hemolysis (the presence of schistocytes on blood smear), thrombocytopenia, and subsequent renal failure should confirm the diagnosis of hemolytic-uremic syndrome. Although Henoch-Schönlein purpura can manifest as abdominal pain and bloody stools, it is not usually associated with colitis. Behçet disease can be distinguished by its typical features (Chapter 155). Other considerations are radiation proctitis, viral colitis in immunocompromised patients, and ischemic colitis (Table 328-4). In infancy, dietary protein intolerance can be confused with ulcerative colitis, although the former is a transient problem that resolves on removal of the offending protein, and ulcerative colitis is extremely rare in this age group. Hirschsprung disease can produce an enterocolitis before or within months after surgical correction; this is unlikely to be confused with ulcerative colitis.
Table 328-4 CHRONIC INFLAMMATORY-LIKE INTESTINAL DISORDERS
INFECTION (SEE TABLE 328-3)
AIDS-ASSOCIATED
IMMUNE-INFLAMMATORY
VASCULAR-ISCHEMIC DISORDERS
OTHER
SLE, systemic lupus erythematosus.
Diagnosis
The diagnosis of ulcerative colitis or ulcerative proctitis requires a typical presentation in the absence of an identifiable specific cause (see Tables 328-3 and 328-4) and typical endoscopic and histologic findings (see Table 328-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree