Ovulatory dysfunction and anovulation affect 15 to 25% of all infertile couples seeking therapy. The ultimate proof of normal ovulation is a subsequent pregnancy, but several other pieces of evidence can be used to confirm the presence or absence of normal ovulation. The process leading to ovulation is composed of several components; disruption of any of these can impede ovulation or the capacity of a mature oocyte to fertilize. Treatment of ovulation disorders remains one of the most successful of all infertility treatments.
Evaluation of Ovulatory Function
History
The first and arguably most telling piece of information about ovulatory function is a thorough menstrual history. Generally speaking, regular menstrual cycles between 28 and 35 days are ovulatory. Shorter or longer cycles may signify anovulation; in shorter cycles, this may be representative of a shortened follicular phase seen in aging, and longer cycles may belie an inadequate luteal phase resulting in an endometrium unreceptive to an embryo. A patient who reliably reports very regular 28-day menstrual cycles is unlikely to have anovulation. On the other hand, a woman who reports infrequent, irregular menses and no moliminal symptoms (or who gives an unclear history of her menstrual cycles) is likely oligo- or anovulatory and should be evaluated.
Background: Physiology of Normal Ovulation
A review of the normal hormonal events that regulate the ovulatory process is essential to an understanding of the tests devised for ovulation detection. The trophic effects of both FSH and luteinizing hormone (LH) result in maturation of ovarian follicles. Release of both FSH and LH from the anterior pituitary is controlled by gonadotropin-releasing hormone (GnRH), which is produced in the hypothalamus in a pulsatile fashion. The ovulatory process is characterized by a rapid midcycle rise in LH that culminates in the LH peak. A consensus from previous reports places the onset of the serum LH surge approximately 36 to 38 hours before ovulation.
20 FSH also increases midcycle but to a lesser degree than LH. The luteal phase is characterized by a rise in the concentration of progesterone produced by the corpus luteum, with maximal concentration reached about 8 days after the LH peak. The menstrual cycle length is variable, with the variation residing in the follicular phase. The length of the luteal phase is consistently 14 days.
Midluteal Serum Progesterone Testing
Detection of serum progesterone greater than 3 ng/mL on day 21 suggests the presence of a functioning corpus luteum, indicating that ovulation occurred.
21 One report indicated that midluteal progesterone levels greater than 8.8 ng/mL were present in more than 95% of spontaneous conception cycles.
22 Given the large variability in progesterone secretion throughout the luteal phase in normal patients, it is controversial whether a higher level of progesterone indicates a “better” ovulation than a lower level.
Urinary Luteinizing Hormone Testing
Detection of an LH surge, suggesting that ovulation is imminent, allows the patient to appropriately time intercourse.
23 There is a 4- to 6-hour lag between serum and urinary LH surges. More than 90% of ovulation episodes can be detected by a single urinary test performed midafternoon or early evening of the day of the surge. This helps couples to define the interval in which conception is most likely (the 2 days prior to ovulation and the third day or day of ovulation).
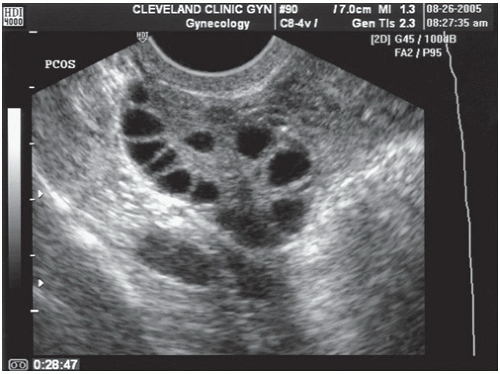
24 Accuracy, reliability, and ease of use vary among the different LH detector kits. These kits are helpful for women in whom ovulation is known to occur; false positives may result in women with polycystic ovarian syndrome (PCOS), premature ovarian failure, and menopause—all conditions associated with increased LH levels.
Basal Body Temperature Charting
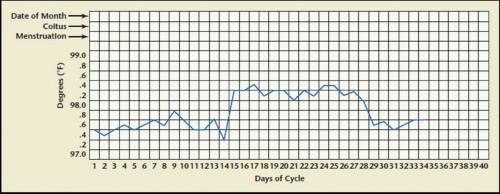
Basal body temperature (BBT) charting has long been used because of its simplicity. The patient is asked to record her body temperature first thing in the morning from the same site each day throughout her menstrual cycle and chart it on a BBT chart (
Fig. 11.1).
The increases in progesterone associated with ovulation will lead to an increase in BBT in the days following ovulation. Biphasic patterns are characteristic in ovulatory cycles; monophasic recordings or a grossly short interval of luteal phase temperature elevation (less than 11 days) may identify patients with absent or poor quality ovulatory function. Challenges to the use of this technique are its need for a special basal body thermometer where differences of one-tenth of a degree may be easily discerned, its inconvenience (the woman must take her temperature first thing upon awakening and doing
anything such as urinating, eating or drinking, and variability—some ovulatory women may exhibit monophasic BBT patterns), and the test cannot reliably define the time of ovulation.
25 The temperature rise seen with ovulation starts 1 to 2 days after the LH surge and lasts for 10 or more days, but because ovulation has already occurred once the increase is seen, it is too late to use the BBT chart for timing sexual intercourse for conception.
Endometrial Biopsy
Histologic evidence of secretory endometrium detected by endometrial biopsy is indicative of ovulation and corpus luteum formation. The endometrial biopsy is performed in the midluteal phase, around 7 days prior
to the expected period. Although the test has been used to diagnose “luteal phase deficiency” in the past, experts have moved away from this concept because of a lack of intra- and interobserver consistency; and the test is unable to provide further useful information other than the presence of ovulation.
26 Further disadvantages of endometrial sampling include expense, patient discomfort, and the known risks of the procedure.
Transvaginal Ultrasound Monitoring
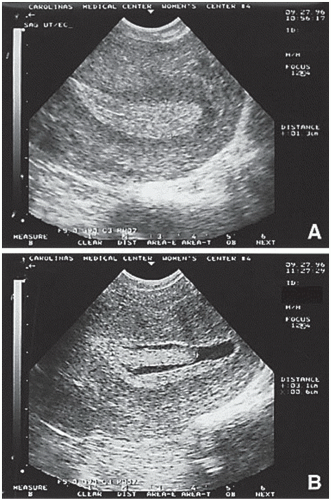
The use of serial transvaginal sonography is a reliable technique to monitor follicular development. Ovulation is deemed to have occurred if the follicle reaches a mean diameter of 18 to 25 mm and subsequently changes in sonographic density or demonstrates sonographic evidence of follicular collapse. However, its use as an initial diagnostic test for all infertile women is not cost-effective.
Other Evaluation: Special Tests to Determine the Underlying Etiology
Once oligo- or anovulation has been diagnosed, additional tests aim to identify the etiology and help choose the optimal treatment. The most common causes of ovulatory dysfunction are PCOS, thyroid dysfunction, hyperprolactinemia, and late-onset congenital adrenal hyperplasia. Therefore, evaluation for PCOS should be undertaken as well as determinations of serum TSH, prolactin, and 17-hydroxyprogesterone.
Prior to instituting treatment for oligo- or anovulation, the patient undergoes ovarian reserve testing, which includes the measurement of an early follicular phase serum FSH and estradiol level. As women age, changes in the patterns and levels of gonadotropin release occur. The follicular phase shortens, and this is associated with an increase in early follicular phase serum FSH prior to any noticeable changes in peak estradiol or progesterone levels or changes in the luteal phase. A high level of FSH on day 3 (e.g., greater than 10 mIU/mL) of the cycle is associated with a poor response to in vitro fertilization (IVF), although a single measurement may not be sufficient—usually two or more high levels in different cycles is required to truly be reliable as a prognostic indicator for IVF response. It is important to note that different FSH assays may yield large variations in measurements even in the same blood sample, so it is important that providers be aware of which assay is being used if specimens go to different labs or try to send all specimens to the same lab for analysis with the same assay.
A high serum estradiol (e.g., greater than 80 pg/mL) is also associated with a poor prognosis for pregnancy after IVF and is usually associated with accelerated, premature follicle recruitment and a reduction in available oocytes. Like FSH measurements, estradiol levels vary widely with different assays.
Other tests for ovarian reserve include anti-Müllerian hormone (AMH) levels, inhibin B levels, clomiphene citrate challenge test (CCCT), exogenous FSH ovarian reserve test, and the use of ultrasound to determine antral follicle count or ovarian volume.
Treatment of Anovulatory Infertility
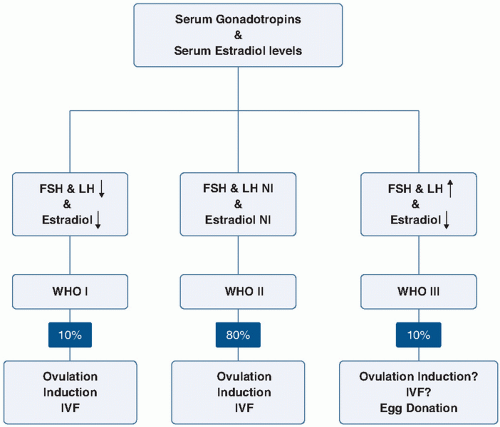
WHO has classified anovulation into three main groups (
Fig. 11.2;
Table 11.2). Women in WHO Group I have hypogonadotropic hypogonadism or hypothalamic amenorrhea. Common causes of this include central nervous system disorders such as hypothalamic or pituitary diseases, stress, eating disorders, and exercise-induced ovulatory dysfunction. Eighty percent of patients with ovulatory dysfunction will have estradiol and gonadotropin levels in the normal range and fall into the category of WHO II, eugonadotropic ovulatory dysfunction.
27 (Group II is discussed in more detail later.) Group III includes hypergonadotropic hypogonadism which may be due to primary ovarian insufficiency, commonly referred to as premature ovarian failure, or to gonadal dysgenesis. The WHO also recognizes that hyperprolactinemia anovulation is a separate category, and usually the gonadotropins are normal or decreased in these cases.
Group I patients often have low body mass index (BMI) (less than 17 kg/m2) resulting from eating disorders or excessive exercise. Mental and emotional stress may disrupt the pulsatile release of GnRH, which leads to a reduction in pituitary secretion of gonadotropin(s) and impairs ovarian function. Treatment usually involves a modification of diet, weight gain, and/or a reduction in exercise, although these approaches are not always well accepted or adhered to by patients. In patients who fail to respond to lifestyle changes, pulsatile GnRH therapy may be an option, and although it is approved for use in the United States, it is not currently available.
The majority of patients in the WHO II category have PCOS.
14 PCOS is characterized by chronic anovulation, hyperandrogenism, and insulin resistance. Diagnostic guidelines vary according to the Rotterdam criteria, the National Institutes of Health, and the Androgen Excess Society, but all include evidence of oligo- or amenorrhea and clinical or biochemical evidence of hyperandrogenism.
28The first line of intervention should be lifestyle change when appropriate because interventions that reduce circulating insulin levels in women with PCOS may restore normal reproductive endocrine function. In patients with BMI greater than 30 kg/m2, weight loss and exercise is the initial recommended treatment for this etiology of anovulation.
If lifestyle intervention alone is ineffective, the first-line agent for ovulation induction in PCOS is clomiphene citrate (CC), an estrogen antagonist that increases gonadotropin release. Clomiphene is usually well tolerated; side effects include hot flushes, mood swings and (rarely) visual changes, and ovarian hyperstimulation syndrome.
29 Available evidence suggests that approximately half of the patients will ovulate on an initial CC regimen of 50 mg for 5 days starting on cycle day 3, 4, or 5 (cycle day 1 is the first day of menstruation). If the patient does not respond, the dose may be increased to 100 mg or even 150 mg.
14 In patients with very irregular menses, micronized progesterone (200 mg daily for 10 days) or medroxyprogesterone acetate (10 mg daily for 10 days) can be given and clomiphene started on cycle day 3 after initiation of the withdrawal bleed. Documentation of ovulation is made with urinary LH monitoring and/or a luteal progesterone level. If clomiphene is taken on cycle days 3 through 7, ovulation usually occurs between days 12 and 15. Typically, each dosage is tried during one menstrual cycle, with an increase in the dose for the next cycle, but recent evidence suggests that patients may be treated with a “stair-step protocol,” during which the dose is increased during the same ovulation induction cycle, if no follicular activity is noted on ultrasound.
30If CC is not successful, the treatment choices include adjunctive use of insulin-sensitizing agents, aromatase inhibitors, gonadotropin therapy, and laparoscopic ovarian diathermy.
Insulin-sensitizing agents such as metformin (Glucophage) have been shown to increase the frequency of spontaneous ovulation, menstrual cyclicity, and the ovulatory response to clomiphene.
31,32 Many women with PCOS who are resistant to CC have demonstrable insulin resistance and hyperinsulinemia. Insulin-sensitizing agents taken alone or in combination with CC can restore ovulation. A recent multicenter trial was designed to study the question whether clomiphene, metformin, or both together should be the first-line therapy in patients with PCOS and infertility.
33 Live-birth rates were 22.5% (47 of 209 subjects) in the clomiphene group, 7.2% (15 of 208) in the metformin group, and 26.8% (56 of 209) in the combination-therapy group (
p <0.001 for metformin versus both clomiphene and combination therapy;
p = 0.31 for clomiphene versus combination therapy). Most experts therefore recommend that clomiphene alone should be the first-line therapy for anovulation in PCOS, with metformin added if there is evidence of hyperinsulinemia or for clomiphene-resistant patients.
Aromatase inhibitors such as letrozole block the conversion of androgens to estrogens and therefore increase gonadotropin concentrations by reducing the negative feedback on the pituitary gland. Letrozole has similar efficacy in ovulation induction as clomiphene, as demonstrated in multiple trials and meta-analyses.
34 However, a study from Canada presented as an abstract in 2005 raised concerns over its safety with respect to cardiac malformations, which led to a warning from the manufacturer of the medication. Despite the fact that subsequent studies demonstrated a comparable safety profile of the medication compared to clomiphene,
35 practitioners tend to still be cautious about the use of letrozole for ovulation induction.
Treatment with injectable gonadotropins with or without intrauterine insemination (IUI) requires close hormonal and sonographic monitoring, is costly, and has significant potential to result in multiple gestation. Laparoscopic destruction of ovarian follicles by laser or cautery has similar success rates as gonadotropin treatment but with lower multiple pregnancy rates.
36 However, risks of the procedure include the formation of adnexal adhesions and a decrease in ovarian reserve. Therefore, this procedure is usually limited to patients for whom other alternatives have been exhausted and patients who do not have access to the most costly but also most effective treatment for clomiphene-resistant PCOS patients, namely IVF.
Premature ovarian failure is defined as the development of primary hypogonadism in a woman prior to the age of 40 years. Some of these women may experience
intermittent ovulation, and 5 to 10% may conceive and deliver.
37 Premature ovarian insufficiency may be due to a variety of causes, including chromosomal defects such as Turner syndrome, fragile X permutation carriers, galactosemia, radiation exposure, certain drug exposures, and autoimmune disease. Despite a growing list of mutation associated with premature ovarian insufficiency, the etiology is still unknown in 75 to 90% of cases.
38In about 3% of women, the primary ovarian failure may precede the development of an autoimmune adrenal insufficiency, a potentially fatal disorder, by several years. Proper screening with serum anti-adrenal and anti-21 hydroxylase antibodies is recommended for these patients.
39 These patients are also at increased risk for developing autoimmune hypothyroidism and should be screened for this as well. Although ultrasound studies indicate that follicular development occurs frequently in these women, ovulation is infrequent.
40 Because spontaneous pregnancy rates are low in this population, treatment options include IVF with oocyte donation and adoption. The evidence to support the use of exogenous estrogen, gonadotropin therapy (with estrogen or with GnRH agonist) are fairly limited and not overly encouraging.
41,
42,
43,
44 Success rates for the use of IVF with oocyte donation depend primarily on the age of the oocyte donor.
In patients with elevated prolactin levels, anovulation is secondary to decreased estradiol concentrations. One isolated increased prolactin level should be confirmed by repeat measurement in the early morning, avoiding recent stress or recent breast or pelvic exams, which can cause mild elevations. If the value is confirmed, hypothyroidism should be ruled out, and magnetic resonance imaging is indicated to rule out a pituitary adenoma. Treatment of hyperprolactinemic anovulation, even in the presence of a pituitary adenoma, is usually by means of dopaminergic drugs, such as bromocriptine (Parlodel). Bromocriptine is an ergot alkaloid derivative with dopamine receptor agonist activity that directly inhibits prolactin secretion.
For ovulation induction, bromocriptine is usually started at 1.25 mg orally at bedtime for 1 week and then increased to 2.5 mg twice daily. After 1 week on this dose (2.5 mg), ovulatory status should be evaluated. In the absence of ovulation, the dose can be increased in 1.25-mg increments (the maximum dosage should not exceed 100 mg/day).
45 After ovulation is established, the medication is maintained until the patient becomes pregnant. Bromocriptine is usually discontinued after a positive pregnancy test but can be used safely during pregnancy. Most patients who conceive do so within six ovulatory cycles with the average being two cycles.
46 Side effects of bromocriptine include nausea, headache, and faintness due to orthostatic hypotension. These effects are minimized by gradually increasing the dose. Taking it with food is recommended to avoid gastrointestinal side effects. Vaginal administration of bromocriptine has been associated with fewer side effects but with similar effectiveness.
47Another dopamine agonist, cabergoline (Dostinex), given once a week, is more effective in normalizing prolactin and restoring menses than bromocriptine and significantly better tolerated. However, given the fact that less data exist on safety in pregnancy, it has not been widely accepted as first-line therapy for ovulation induction in hyperprolactinemic patients seeking fertility.
48