Infertility and Assisted Reproductive Technologies
Cindy M. P. Duke
Mindy S. Christianson
INFERTILITY
Definitions
Infertility: failure of a couple of reproductive age to conceive after at least 1 year of regular coitus without contraception
Primary infertility: infertility in a woman who has never been pregnant
Secondary infertility: infertility in a woman who has had one or more previous pregnancies
Fecundability: probability of achieving pregnancy within one menstrual cycle. For a normal couple, this is approximately 25%.
Fecundity: ability to achieve a live birth within one menstrual cycle
Incidence
Data from the 2002 National Survey of Family Growth (NSFG) revealed that 2% of women of reproductive age in the United States had an infertility-related medical appointment within the past year.
Data from the 2006 to 2010 NSFG continued to show that as in the 2002 data, 11.9% of women of reproductive age reported having received infertility services at some point in their lives.
Additionally, 6% of couples with women of reproductive age reported not becoming pregnant after not using contraception in the prior year.
TABLE 35-1 Differential Diagnosis of Infertility
Diagnosis
Percent
Basic Evaluation
Male factors
30
Semen analysis
Tubal/uterine/peritoneal factors
25
HSG, laparoscopy, chromopertubation
Anovulation/ovarian factors
25
BBT chart, midluteal progesterone level, endometrial biopsy, luteinizing hormone testing
Cervical factors
10
Postcoital test
Unexplained infertility
10
All of the above
HSG, hysterosalpingogram; BBT, basal body temperature. Adapted from Fritz MA. Infertility. In Fritz MA, Speroff L, eds. Clinical Gynecologic Endocrinology and Infertility, 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010:1137-1190.
Demand for infertility services has increased in recent years. Reasons include the following:
Delayed childbearing in women due to career demands and marriage at a later age
An increase in variety and effectiveness of assisted reproductive technology (ART) treatments and an increased public awareness of these treatments, including in vitro fertilization (IVF)
An increase in tubal factor infertility as a consequence of sexually transmitted diseases
Relative scarcity of babies placed for adoption due to effective contraception and increased availability of abortion services.
Differential Diagnosis
The differential diagnosis of infertility encompasses five principal categories (Table 35-1):
Male factor
Ovulatory dysfunction
Structural (tubal/peritoneal and uterine)
Cervical factors
Unexplained causes
Coital factors
EVALUATION
Evaluation is indicated for women who fail to conceive after 1 or more years of regular, unprotected intercourse.
Women older than the age of 35 years should be evaluated sooner (i.e., after 6 months of regular, unprotected intercourse).
Successful reproduction requires proper structure and function of the entire reproductive axis, including hypothalamus, pituitary gland, ovaries, fallopian tube, uterus, cervix, and vagina.
Infertility evaluation comprises six major elements:
History and physical examination
Semen analysis
Assessment of ovarian reserve
Tests for occurrence of ovulation
Tests to evaluate for structural abnormalities: These include evaluation of tubal patency, detection of uterine abnormalities, and determination of peritoneal abnormalities.
Sperm—cervical mucus interaction (postcoital testing [PCT])—for select patients. This has fallen out of favor and is infrequently used.
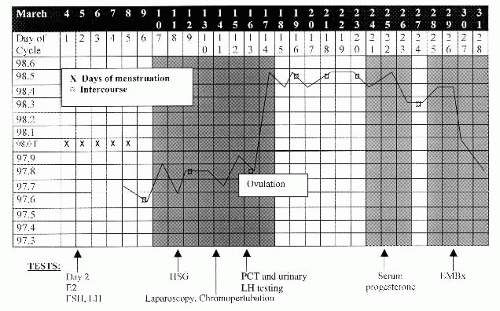
With proper coordination, the evaluation can be completed within one menstrual cycle (Fig. 35-1). No abnormality or cause of infertility is identified in 10% to 15% of couples. This group comprises a category known as “unexplained infertility.”
History and Physical Examination
The initial assessment involves an extended and complete history from both partners and a complete physical examination.
Physical examination of the male partner can be deferred pending the results of the semen analysis. Abnormal results of a semen analysis warrants referral to a urologist.
History elicited from both male and female partners should include the following:
Duration of infertility, methods of contraception, previous evaluation and treatment, prior reproductive history, sexual dysfunction, coital frequency and satisfaction, sexually transmitted infections, tobacco and alcohol use, caffeine use, family history of mental retardation, and birth defects
History elicited from the female partner should include the following:
Complete menstrual history, dysmenorrhea or menorrhagia, pelvic or abdominal pain, dyspareunia, symptoms of thyroid disease, galactorrhea, symptoms of hirsutism, exercise habits, and indices of stress
Components of the female physical exam should include the following:
Weight and body mass index, thyroid exam, breast exam, signs of hirsutism, pelvic or abdominal tenderness, uterine size and mobility, adnexal masses and/or tenderness, cul-de-sac tenderness, or nodularity
Baseline studies and labs may include the following: thyroid-stimulating hormone, prolactin, follicle-stimulating hormone (FSH), 17-hydroxyprogesterone, serum testosterone, progesterone, dehydroepiandrosterone (DHEAS), semen analysis, and hysterosalpingogram (HSG).
Male Factor Infertility Evaluation
The semen analysis is the cornerstone of male factor infertility evaluation.
Semen sample should be collected after at least 48 to 72 hours abstinence and is best evaluated within 1 hour of ejaculation.
Obtained either by masturbation or by sexual intercourse with a silicone condom because latex condoms are spermicidal.
Lower reference limits (normal parameters) according to the World Health Organization (WHO) are as follows:
Ejaculate volume of at least 1.5 mL
Semen pH above 7.2
Sperm concentration of at least 20 million/mL
Greater than 40% total motility; greater than 32% progressive motility
Greater than 4% normal morphology
Semen analysis terminology:
Azoospermia: absence of sperm in the ejaculate
Oligospermia: a concentration of fewer than 20 million sperm/mL
Asthenospermia: reduced sperm motility
Men with an abnormal semen analysis should be referred to a urologist, especially in cases of oligospermia or azoospermia. Causes of male factor infertility include the following:
Klinefelter syndrome
Karyotype is 47, XXY.
Most common genetic anomaly in azoospermic men
Found in 1:500 to 1:1,000 live male births
Incidence: 3% of infertile men, 3.5% to 14.5% of azoospermic men, 1% of couples referred to intracytoplasmic sperm injection (ICSI)
Congenital absence of the vas deferens (CAVD)
Associated with cystic fibrosis gene mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.
Partners of men with CAVD must be tested for the CFTR gene mutation before pursuing infertility treatment with retrieved sperm.
Y-chromosome microdeletions
May be found in up to 7% of men with male factor infertility
Although these men may be able to father children via IVF/ICSI, male offspring will inherit the Y-chromosome microdeletion and be infertile.
EXCLUSION OF OVULATORY FACTOR INFERTILITY
To exclude ovulatory dysfunction, the presence of ovulation must be confirmed. In addition, ovarian reserve should be assessed to exclude oocyte depletion and premature ovarian failure.
Confirmation of Ovulation
The basal body temperature (BBT) chart (see Fig. 35-1) is a simple means of determining whether ovulation has occurred.
The woman’s temperature is taken daily on awakening, before any activity, and recorded on a graph.
After ovulation, rising progesterone levels increase the basal temperature by approximately 0.4°F (0.22°C) through a hypothalamic thermogenic effect.
Because the rise in progesterone may occur anytime from 2 days before ovulation to 1 day after, the temperature elevation does not predict the exact moment of ovulation but offers retrospective confirmation of its occurrence.
A temperature elevation is usually sustained for 14 ± 2 days. One that persists for <11 days is suggestive of a luteal phase defect.
Midluteal phase progesterone level is another test to assess ovulation.
A concentration >3.0 ng/mL in a blood sample drawn between days 19 and 23 suggests ovulation has occurred. Normal adequate luteal support usually produces a progesterone concentration >10 ng/mL.
Daily monitoring of urinary luteinizing hormone (LH) is now widely used, given the proliferation of commercial tests for home use.
Using a threshold concentration of 40 mIU/mL, positive testing for urinary LH correlates well with the surge of serum LH levels that trigger ovulation.
Assessment of Ovarian Reserve
Depleted ovarian reserve adversely impacts fecundability given the inferior quantity and quality of remaining oocytes. The following tests help identify both a depleted reserve and the likelihood of response to controlled ovarian hyperstimulation (COH) during assisted reproduction:
Day 3 FSH concentration: Values below 10 to 15 mIU/mL suggest adequate ovarian reserve. The exact cutoff depends on the particular laboratory reference standards.
Measurement of anti-müllerian hormone (AMH) levels can also be helpful in predicting ovarian reserve. AMH is a measure of the primordial follicle pool and over a woman’s reproductive lifetime steadily decreases to undetectable levels by menopause. There are kits available to assay for this hormone and the exact cutoff depends on the specific laboratory/assay reference standard.
Imaging of antral follicle counts by ultrasonography
Clomiphene citrate challenge test: The administration of clomiphene citrate 100 mg orally on menstrual cycle days 5 to 9 with measurement of day 3 and day 10 FSH. An exaggerated FSH response portends poorly for spontaneous or assisted conception.
EXCLUSION OF STRUCTURAL FACTORS (TUBAL/PERITONEAL AND UTERINE)
Tubal/peritoneal factors include endometriosis, pelvic adhesion disease, or previous bilateral tubal ligation. Uterine factors include leiomyomata, intrauterine synechiae (Asherman syndrome), septae, and other müllerian anomalies.
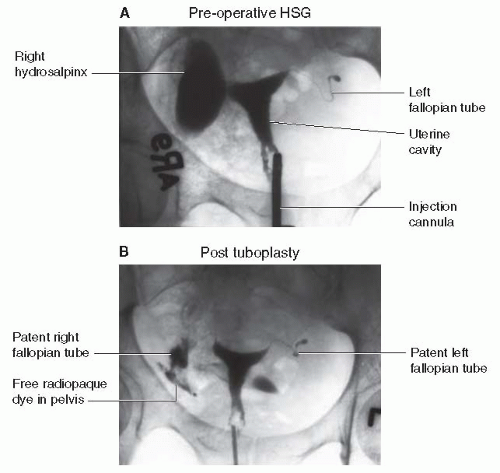
HSG assesses uterine and fallopian tube contour and tubal patency (Fig. 35-2).
HSG shows appreciable müllerian anomalies as well as most endometrial polyps, synechiae, and submucosal fibroids. It can also determine tubal patency.
Performed in the early follicular phase, within 1 week of cessation of menstrual flow, to minimize chances of interrupting a pregnancy
The procedure is performed by injecting a radiopaque dye through the cervix. As more dye is injected, the dye normally passes through the uterine cavity into the fallopian tubes and then spills into the peritoneal cavity.
X-ray films are taken under fluoroscopy to evaluate tubal patency.
Nonsteroidal anti-inflammatory drugs may be given to prevent cramping.
HSG may have therapeutic effects. Several studies have indicated increased pregnancy rates for several months after the procedure.
Prophylactic antibiotics (doxycycline, 100 mg orally twice daily for 5 to 7 days) are advisable when the patient has a history of pelvic inflammatory disease or when hydrosalpinges are identified during the study.
Saline infusion ultrasonography (sonohysterography [SHG])
SHG involves transvaginal ultrasound after the introduction of sterile water or saline into the uterine cavity.
Useful in assessment of uterine cavity abnormalities such as polyps or submucosal fibroids
Hysteroscopy
Definitive method to evaluate the uterine cavity
Reserved for those patients with HSG or SHG results that merit further evaluation. It offers the possibility of minimally invasive treatment at time of the procedure
Diagnostic laparoscopy
Assesses peritoneal and tubal factors, such as endometriosis and pelvic adhesions and can provide access for simultaneous corrective surgery
Laparoscopy should be scheduled in the follicular phase. This is the final and most invasive step in the patient’s evaluation.
Findings on HSG correlate with laparoscopic findings 60% to 70% of the time.
Chromopertubation: dye (usually a dilute solution of indigo carmine) instilled through the fallopian tubes during laparoscopy to visually document tubal patency
Hysteroscopy may also be included to ensure that no intrauterine abnormalities were missed on the HSG.
EXCLUSION OF CERVICAL FACTOR INFERTILITY
The PCT or Huhner test allows direct analysis of sperm and cervical mucus interaction and provides a rough estimate of sperm quality.
The test is done between days 12 and 14 of a 28- to 30-day menstrual cycle (after 48 hours of abstinence) when maximum estrogen secretion is present and the mucus is examined within 2 to 12 hours.
Because interpretation of the PCT is subjective, the validity of the test is controversial, despite its long history of use.
The test’s use is most valuable for patients with history or physical exam findings suggestive of cervical factor, when the results will help direct treatment. However, a finding of 5 to 10 progressively motile spermatozoa per high-power field and clear acellular mucus with a spinnbarkeit (the degree to which the mucus stretches between two slides) of 8 cm generally suggests normal cervical function.
Fecundity rates do not correlate directly with the number of motile sperm seen. The most common cause of an abnormal PCT is poor timing. Other causes include cervical stenosis, hypoplastic endocervical canal, coital dysfunction, and male factors. The sample can also be assessed for pH, mucus cellularity, white blood cell, and ferning. Clumping and flagellation of sperm without progression are often suggestive of antisperm antibodies.
ENDOMETRIAL BIOPSY AND THE LUTEAL PHASE DEFECT
Endometrial biopsy, historically, can document ovulation by histologic demonstration of decidualized stroma, assess for endometritis, and allows for histologic dating of the endometrium within 2 to 3 days and usually performed between days 24 and 26 of a 28-day menstrual cycle or 2 to 4 days before anticipated menstruation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree