Pregnancy-Induced Immunological Changes
Even after intensive study, many of the maternal immunological adaptations to pregnancy are not well elucidated. It is known that pregnancy is associated with an increase in CD4-positive T cells secreting Th2-type cytokines—for example interleukins 4, 5, 10, and 13. Th1-type cytokine production—for example, interferon gamma and interleukin 2—appears to be somewhat suppressed, leading to a Th2 bias in pregnancy. This bias affects the ability to rapidly eliminate certain intracellular pathogens during pregnancy, although the clinical implications of this suppression are unknown (Jamieson, 2006a; Raghupathy, 2001; Svensson-Arvelund, 2014). Importantly, the Th2 humoral immune response remains intact.
 Fetal and Newborn Immunology
Fetal and Newborn Immunology
The active immunological capacity of the fetus and neonate is compromised compared with that of older children and adults. That said, fetal cell-mediated and humoral immunity begin to develop by 9 to 15 weeks’ gestation (Warner, 2010). The primary fetal response to infection is immunoglobulin M (IgM). Passive immunity is provided by IgG transferred across the placenta. By 16 weeks, this transfer begins to increase rapidly, and by 26 weeks, fetal concentrations are equivalent to those of the mother. After birth, breast feeding is protective against some infections, although this protection begins to decline at 2 months of age. Current World Health Organization (2013) recommendations are to exclusively breast feed for the first 6 months of life with partial breast feeding until 2 years of age.
Vertical transmission refers to passage from the mother to her fetus of an infectious agent through the placenta, during labor or delivery, or by breast feeding. Thus, preterm rupture of membranes, prolonged labor, and obstetrical manipulations may increase the risk of neonatal infection. Those occurring less than 72 hours after delivery are usually caused by bacteria acquired in utero or during delivery, whereas infections after that time most likely were acquired afterward. Table 64-1 details specific infections by mode and timing of acquisition.
TABLE 64-1. Specific Causes of Some Fetal and Neonatal Infections
Intrauterine
Transplacental
Viruses: varicella-zoster, coxsackie, human parvovirus B19, rubella, cytomegalovirus, HIV
Bacteria: Listeria, syphilis, Borrelia
Protozoa: toxoplasmosis, malaria
Ascending infection
Bacteria: group B streptococcus, coliforms
Viruses: HSV
Intrapartum
Maternal exposure
Bacteria: gonorrhea, chlamydia, group B streptococcus, tuberculosis, mycoplasmas
Viruses: HSV, HPV, HIV, hepatitis B, hepatitis C
External contamination
Bacteria: staphylococcus, coliforms
Viruses: HSV, varicella zoster
Neonatal
Human transmission: staphylococcus, HSV
Respirators and catheters: staphylococcus, coliforms
Neonatal infection, especially in its early stages, may be difficult to diagnose because neonates often fail to express classic clinical signs. If the fetus was infected in utero, there may be depression and acidosis at birth for no apparent reason. The neonate may suck poorly, vomit, or show abdominal distention. Respiratory insufficiency may develop, which may present similarly to idiopathic respiratory distress syndrome. The neonate may be lethargic or jittery. The response to sepsis may be hypothermia rather than hyperthermia, and the total leukocyte and neutrophil counts may be depressed.
VIRAL INFECTIONS
 Varicella-Zoster Virus
Varicella-Zoster Virus
Several viruses that infect the mother can also cause devastating fetal infections. Varicella-zoster virus (VZV) is a double-stranded DNA herpes virus acquired predominately during childhood, and 95 percent of adults have serological evidence of immunity (Plourd, 2005). The incidence of adult varicella infections declined by 74 percent after the introduction of varicella vaccination, probably secondary to herd immunity (Marin, 2008). This has resulted in a decrease in maternal and fetal varicella infections (Khandaker, 2011). Primary infection—varicella or chicken pox—is transmitted by direct contact with an infected individual, although respiratory transmission has been reported. The incubation period is 10 to 21 days, and a nonimmune woman has a 60- to 95-percent risk of becoming infected after exposure (Whitley, 2012). She is then contagious from 1 day before the onset of the rash until the lesions are crusted over.
Maternal Infection
Primary varicella infection presents with a 1- to 2-day flu-like prodrome, which is followed by pruritic vesicular lesions that crust over in 3 to 7 days. Infection tends to be more severe in adults, and a quarter of varicella deaths are within the 5 percent of nonimmune adults (Centers for Disease Control and Prevention, 2007).
Mortality is predominately due to varicella pneumonia, which is thought to be more severe during adulthood and particularly in pregnancy. Between 5 and 20 percent of infected pregnant women developed pneumonitis (Centers for Disease Control and Prevention, 2007; Harger, 2002). Risk factors for VZV pneumonia include smoking and having more than 100 cutaneous lesions. Maternal mortality rates with pneumonia have decreased to 1 to 2 percent (Chandra, 1998). Symptoms of pneumonia usually appear 3 to 5 days into the course of illness. It is characterized by fever, tachypnea, dry cough, dyspnea, and pleuritic pain. Nodular infiltrates are similar to other viral pneumonias (Chap. 51, p. 1018). Although resolution of pneumonitis parallels that of skin lesions, fever and compromised pulmonary function may persist for weeks.
If primary varicella infection is reactivated years later, it causes herpes zoster or shingles (Whitley, 2012). This presents as a unilateral dermatomal vesicular eruption associated with severe pain. Zoster does not appear to be more frequent or severe in pregnant women. Enders and associates (1994) reviewed 366 cases during pregnancy and found little evidence that zoster causes congenital malformations. Zoster is contagious if blisters are broken, although less so than primary varicella infection.
Fetal and Neonatal Infection
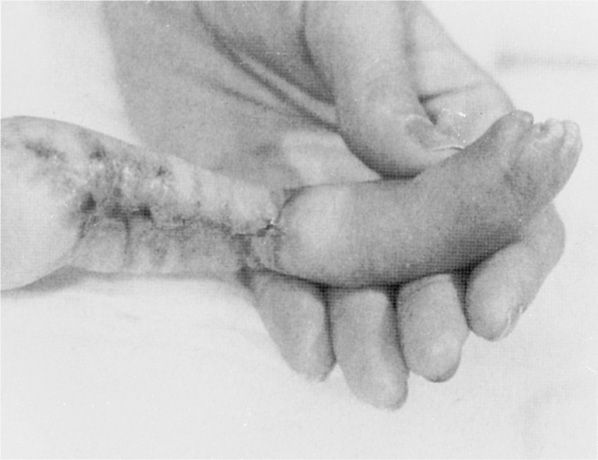
In women with chicken pox during the first half of pregnancy, the fetus may develop congenital varicella syndrome. Some features include chorioretinitis, microphthalmia, cerebral cortical atrophy, growth restriction, hydronephrosis, limb hypoplasia, and cicatricial skin lesions as shown in Figure 64-1 (Auriti, 2009). Enders and coworkers (1994) evaluated 1373 pregnant women with varicella infection. When maternal infection developed before 13 weeks, only two of 472 pregnancies—0.4 percent—had neonates with congenital varicella. The highest risk was between 13 and 20 weeks, during which time seven of 351 exposed fetuses—2 percent—had evidence of congenital varicella. After 20 weeks’ gestation, the researchers found no clinical evidence of congenital infection. Thus, congenital infections, particularly after 20 weeks, are uncommon. Subsequent sporadic reports have described central nervous system abnormalities and skin lesions in fetuses who developed congenital varicella in weeks 21 to 28 of gestation (Lamont, 2011b; Marin, 2007).
FIGURE 64-1 Atrophy of the lower extremity with bony defects and scarring in a fetus infected during the first trimester by varicella. (From Paryani, 1986, with permission.)
If the fetus or neonate is exposed to active infection just before or during delivery, and therefore before maternal antibody has been formed, then there is a serious threat to newborns. Attack rates range from 25 to 50 percent, and mortality rates approach 30 percent. In some instances, neonates develop disseminated visceral and central nervous system disease, which is commonly fatal. For this reason, varicella-zoster immune globulin should be administered to neonates born to mothers who have clinical evidence of varicella 5 days before and up to 2 days after delivery.
Diagnosis
Maternal varicella infection is usually diagnosed clinically. The virus may also be isolated by scraping the vesicle base during primary infection and performing a Tzanck smear, tissue culture, or direct fluorescent antibody testing. Also, available nucleic acid amplification tests (NAATs) are very sensitive. Congenital varicella may be diagnosed using NAAT analysis of amnionic fluid, although a positive result does not correlate well with the development of congenital infection (Mendelson, 2006). A detailed anatomical sonographic evaluation performed at least 5 weeks after maternal infection may disclose abnormalities, but the sensitivity is low (Mandelbrot, 2012).
Management
Maternal Viral Exposure. There are several aspects of maternal varicella virus exposure and infection in pregnancy that affect management. Because most adults are VZV seropositive, exposed pregnant women with a negative history for chicken pox should undergo VZV serologic testing. At least 70 percent of these women will be seropositive, and thus immune. Exposed pregnant women who are susceptible should be given VariZIG, a recently approved varicella zoster immune globulin. Although best given within 96 hours of exposure, its use is approved for up to 10 days to prevent or attenuate varicella infection (Centers for Disease Control and Prevention, 2012a, 2013h).
Maternal Infection. Any patient diagnosed with primary varicella infection should be isolated from pregnant women. Because pneumonia often presents with few symptoms, a chest radiograph is recommended by many. Most women require only supportive care, but those who require intravenous (IV) fluids and especially those with pneumonia are hospitalized. Intravenous acyclovir therapy is given to women requiring hospitalization—500 mg/m2 or 10 to 15 mg/kg every 8 hours.
Vaccination. An attenuated live-virus vaccine—Varivax—was approved in 1995. Two doses, given 4 to 8 weeks apart, are recommended for adolescents and adults with no history of varicella. This results in 98-percent seroconversion (Centers for Disease Control and Prevention, 2007). Importantly, vaccine-induced immunity diminishes over time, and the breakthrough infection rate approximates 5 percent at 10 years (Chaves, 2007). The vaccine is not recommended for pregnant women or for those who may become pregnant within a month following each vaccine dose. That said, a registry of 981 vaccine-exposed pregnancies reports no cases of congenital varicella syndrome or other congenital associated malformations (Wilson, 2008). The attenuated vaccine virus is not secreted in breast milk. Thus, postpartum vaccination should not be delayed because of breast feeding (American College of Obstetricians and Gynecologists, 2013a; Bohlke, 2003).
 Influenza
Influenza
These respiratory infections are caused by members of the family Orthomyxoviridae. Influenza A and B form one genus of these RNA viruses, and both cause epidemic human disease. Influenza A viruses are subclassified further by hemagglutinin (H) and neuraminidase (N) surface antigens. Influenza outbreaks occur annually, and the most recent epidemic was in 2013–2014 caused by an influenza A/H1N1 strain.
Maternal and Fetal Infection
Maternal influenza is characterized by fever, dry cough, and systemic symptoms. Infection usually is not life-threatening in otherwise healthy adults, but pregnant women appear to be more susceptible to serious complications, particularly pulmonary involvement (Cox, 2006; Neuzil, 1998; Rasmussen, 2012). In early 2003, widespread influenza A infection affected pregnant women. At Parkland Hospital, more than 100 women were hospitalized for this infection, and 12 percent had pulmonary infiltrates seen radiographically (Rogers, 2010).
There is no firm evidence that influenza A virus causes congenital malformations (Irving, 2000; Saxén, 1990). Conversely, Lynberg and colleagues (1994) reported increased neural-tube defects in neonates born to women with influenza early in pregnancy, but this was possibly associated with hyperthermia (Chap. 12, p. 284). Viremia is infrequent, and transplacental passage is rare (Rasmussen, 2012). Stillbirth, preterm delivery, and first-trimester abortion have all been reported, usually correlated to severity of maternal infection (Centers for Disease Control and Prevention, 2011; Pierce, 2011; Yates, 2010).
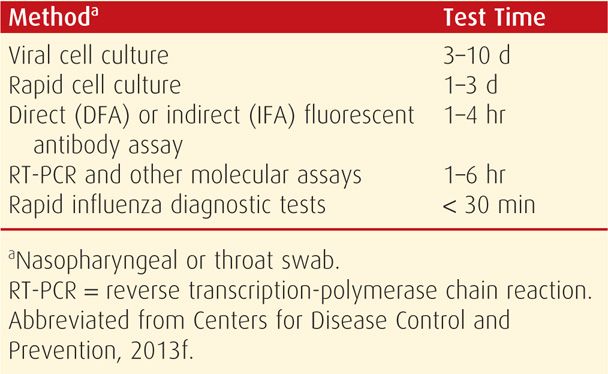
Influenza may be detected in nasopharyngeal swabs using viral antigen rapid detection assays (Table 64-2). Reverse transcriptase–polymerase chain reaction (RT-PCR) is the more sensitive and specific test, although not commercially available in many hospitals (Dolin, 2012). In contrast, rapid influenza diagnostic tests (RIDTs) are least indicative, with sensitivities of 40 to 70 percent. In the Parkland Hospital emergency room, immunofluorescent antibody assays are used. Nasopharyngeal specimens are collected as early as possible after symptom onset to maximize influenza testing sensitivity. Importantly, decisions to administer antiviral medications for influenza treatment or chemoprophylaxis should be based on clinical symptoms and epidemiological factors. Moreover, the start of therapy should not be delayed pending testing results (Centers for Disease Control and Prevention, 2013f).
TABLE 64-2. Outpatient Influenza A and B Virus Testing Methods
Management
Two classes of antiviral medications are currently available. Neuraminidase inhibitors are highly effective for the treatment of early influenza A and B. These include oseltamivir (Tamiflu), which is taken orally for treatment and for chemoprophylaxis, and zanamivir (Relenza), which is inhaled for treatment. Peramivir is an investigational drug administered intravenously.
The adamantanes include amantadine and rimantadine, which were used for years for treatment and chemoprophylaxis of influenza A. In 2005, influenza A resistance to adamantine was reported to be > 90 percent in the United States, and thus, adamantane use is not currently recommended. It is possible that these drugs may again be effective for subsequently mutated strains.
There is limited experience with all five of these antiviral agents in pregnant women. They are Food and Drug Administration category C drugs, used when the potential benefits outweigh the risks. At Parkland Hospital, we recommend starting oseltamivir treatment within 48 hours of symptom onset—75 mg orally twice daily for 5 days. Prophylaxis with oseltamivir, 75 mg orally once daily for 10 days, is also recommended for significant exposures. Antibacterial medications should be added when a secondary bacterial pneumonia is suspected (Chap. 51, p. 1016).
Vaccination
Effective vaccines are formulated annually. Vaccination against influenza throughout the influenza season, but optimally in October or November, is recommended by the Centers for Disease Control and Prevention (CDC) (2013e) and the American College of Obstetricians and Gynecologists (2010) for all women who will be pregnant during the influenza season. This is especially important for those affected by chronic medical disorders such as diabetes, heart disease, asthma, or human immunodeficiency virus (HIV) infection (Jamieson, 2012). Inactivated vaccine prevents clinical illness in 70 to 90 percent of healthy adults. Importantly, there is no evidence of teratogenicity or other adverse maternal or fetal events (Conlin, 2013; Kharbanda, 2013; Munoz, 2012; Nordin, 2013; Sheffield, 2012). Moreover, several studies have found decreased rates of influenza in infants up to 6 months of age whose mothers were vaccinated during pregnancy (Steinhoff, 2012; Zaman, 2008). Immunogenicity of the trivalent inactivated seasonal influenza vaccine in pregnant women is similar to that in the nonpregnant individual (Sperling, 2012). A live attenuated influenza virus vaccine is available for intranasal use but is not recommended for pregnant women.
 Mumps
Mumps
This uncommon adult infection is caused by an RNA paramyxovirus. Because of childhood immunization, up to 90 percent of adults are seropositive (Rubin, 2012). The virus primarily infects the salivary glands, and hence its name—mumps—is derived from Latin, “to grimace.” Infection also may involve the gonads, meninges, pancreas, and other organs. It is transmitted by direct contact with respiratory secretions, saliva, or through fomites. Treatment is symptomatic, and mumps during pregnancy is no more severe than in nonpregnant adults.
Women who develop mumps in the first trimester may have an increased risk of spontaneous abortion. Infection in pregnancy is not associated with congenital malformations, and fetal infection is rare (McLean, 2013).
The live attenuated Jeryl-Lynn vaccine strain is part of the MMR vaccine—measles, mumps, and rubella—and is contraindicated in pregnancy according to the CDC (McLean, 2013). No malformations attributable to MMR vaccination in pregnancy have been reported, but pregnancy should be avoided for 30 days after mumps vaccination. The vaccine may be given to susceptible women postpartum, and breast feeding is not a contraindication.
 Rubeola (Measles)
Rubeola (Measles)
Measles is caused by a highly contagious RNA virus of the family Paramyxoviridae that only infects humans. Annual outbreaks occur in late winter and early spring, transmission is primarily by respiratory droplets, and the secondary attack rate among contacts exceeds 90 percent (Moss, 2012). Infection is characterized by fever, coryza, conjunctivitis, and cough. The characteristic erythematous maculopapular rash develops on the face and neck and then spreads to the back, trunk, and extremities. Koplik spots are small white lesions with surrounding erythema found within the oral cavity. Diagnosis is most commonly performed by serology, although RT-PCR tests are available. Treatment is supportive.
Pregnant women without evidence of measles immunity should be administered intravenous immune globulin (IVIG), 400 mg/kg within 6 days of a measles exposure (McLean, 2013). Active vaccination is not performed during pregnancy. However, susceptible women can be vaccinated routinely postpartum, and breast feeding is not contraindicated (Ohji, 2009).
The virus does not appear to be teratogenic (Siegel, 1973). However, an increased frequency of abortion, preterm delivery, and low-birthweight neonates is noted with maternal measles (American Academy of Pediatrics, 2006; Siegel, 1966). If a woman develops measles shortly before birth, there is considerable risk of serious infection developing in the neonate, especially in a preterm neonate.
 Rubella—German Measles
Rubella—German Measles
This RNA togavirus typically causes infections of minor importance in the absence of pregnancy. Rubella infection in the first trimester, however, poses significant risk for abortion and severe congenital malformations. Transmission occurs via nasopharyngeal secretions, and the transmission rate is 80 percent to susceptible individuals. The peak incidence is late winter and spring.
Maternal rubella infection is usually a mild, febrile illness with a generalized maculopapular rash beginning on the face and spreading to the trunk and extremities. Other symptoms may include arthralgias or arthritis, head and neck lymphadenopathy, and conjunctivitis. The incubation period is 12 to 23 days. Viremia usually precedes clinical signs by about a week, and adults are infectious during viremia and through 5 to 7 days of the rash. Up to half of maternal infections are subclinical despite viremia that may cause devastating fetal infection (McLean, 2013; Zimmerman, 2012).
Diagnosis
Rubella may be isolated from the urine, blood, nasopharynx, and cerebrospinal fluid for up to 2 weeks after rash onset. The diagnosis is usually made, however, with serological analysis. Specific IgM antibody can be detected using enzyme-linked immunoassay from 4 to 5 days after onset of clinical disease, but it can persist for up to 6 weeks after appearance of the rash (Zimmerman, 2012). Importantly, rubella reinfection can give rise to transient low levels of IgM. Serum IgG antibody titers peak 1 to 2 weeks after rash onset. This rapid antibody response may complicate serodiagnosis unless samples are initially collected within a few days after the onset of the rash. If, for example, the first specimen was obtained 10 days after the rash, detection of IgG antibodies would fail to differentiate between very recent disease and preexisting immunity to rubella. IgG avidity testing is performed concomitant with the serological tests above. High-avidity IgG antibodies indicate an infection at least 2 months in the past.
Fetal Effects
Rubella is one of the most complete teratogens, and sequelae of fetal infection are worst during organogenesis (Adams Waldorf, 2013). Pregnant women with rubella infection and a rash during the first 12 weeks of gestation have a fetus with congenital infection in up to 90 percent of cases (Miller, 1982; Zimmerman, 2012). At 13 to 14 weeks’ gestation, this incidence was 54 percent, and by the end of the second trimester, it was 25 percent. Defects are rare after 20 weeks (Miller, 1982). According to Reef and colleagues (2000), congenital rubella syndrome includes one or more of the following:
• Eye defects—cataracts and congenital glaucoma
• Congenital heart defects—patent ductus arteriosus and pulmonary artery stenosis
• Sensorineural deafness—the most common single defect
• Central nervous system defects—microcephaly, developmental delay, mental retardation, and meningoencephalitis
• Pigmentary retinopathy
• Neonatal purpura
• Hepatosplenomegaly and jaundice
• Radiolucent bone disease
Neonates born with congenital rubella may shed the virus for many months and thus be a threat to other infants and to susceptible adults who contact them.
The extended rubella syndrome, with progressive panencephalitis and type 1 diabetes, may not develop clinically until the second or third decade of life. As many as a third of neonates who are asymptomatic at birth may manifest such developmental injury (Webster, 1998).
Management and Prevention
There is no specific treatment for rubella. Droplet precautions for 7 days after the onset of the rash are recommended. Primary prevention relies on comprehensive vaccination programs (Coonrod, 2008). Although large epidemics of rubella have virtually disappeared in the United States because of immunization, up to 10 percent of women in the United States are susceptible (Zimmerman, 2012). Cluster outbreaks during the 1990s mainly involved persons born outside the United States, as congenital rubella is still common in developing nations (Banatvala, 2004; Reef, 2002).
To eradicate rubella and prevent congenital rubella syndrome completely, a comprehensive approach is recommended for immunizing the adult population (McLean, 2013). MMR vaccine should be offered to nonpregnant women of childbearing age who do not have evidence of immunity whenever they make contact with the health-care system. Vaccination of all susceptible hospital personnel who might be exposed to patients with rubella or who might have contact with pregnant women is important. Rubella vaccination should be avoided 1 month before or during pregnancy because the vaccine contains attenuated live virus. Although there is a small overall theoretical risk of up to 2.6 percent, there is no observed evidence that the vaccine induces malformations (Badilla, 2007; McLean, 2013). MMR vaccination is not an indication for pregnancy termination. Prenatal serological screening for rubella is indicated for all pregnant women. Women found to be nonimmune should be offered the MMR vaccine postpartum.
Despite native or vaccine-induced immunity, subclinical rubella maternal reinfection may develop during outbreaks. And although fetal infection can rarely occur, no adverse fetal effects have been described.
 Respiratory Viruses
Respiratory Viruses
More than 200 antigenically distinct respiratory viruses cause the common cold, pharyngitis, laryngitis, bronchitis, and pneumonia. Rhinovirus, coronavirus, and adenovirus are major causes of the common cold. The RNA-containing rhinovirus and coronavirus usually produce a trivial, self-limited illness characterized by rhinorrhea, sneezing, and congestion. The DNA-containing adenovirus is more likely to produce cough and lower respiratory tract involvement, including pneumonia.
Teratogenic effects of respiratory viruses are controversial. Women with a common cold had a four- to fivefold increased risk of fetal anencephaly in a 393-woman cohort in the Finnish Register of Congenital Malformations (Kurppa, 1991). In another population study, Shaw and coworkers (1998) analyzed California births from 1989 to 1991 and concluded that low attributable risks for neural-tube defects were associated with many illnesses in early pregnancy. Recently, amnionic fluid viral PCR studies were performed in 1191 women undergoing amniocentesis for fetal karyotyping. Viral PCR was positive in 6.5 percent, with adenovirus being the virus most frequently identified. There was an association with fetal-growth restriction, nonimmune hydrops, foot/hand abnormalities, and neural-tube defects (Adams, 2012).
Adenoviral infection is a known cause of childhood myocarditis. Towbin (1994) and Forsnes (1998) used PCR tests to identify and link adenovirus to fetal myocarditis and nonimmune hydrops.
 Hantaviruses
Hantaviruses
These RNA viruses are members of the family Bunyaviridae. They are associated with a rodent reservoir, and transmission involves inhalation of virus excreted in rodent urine and feces. An outbreak in the Western United States occurred in 1993 due to Sin Nombre virus. The resulting Hantavirus pulmonary syndrome was characterized by severe adult respiratory distress syndrome with a case-fatality rate of 30 to 40 percent (Peters, 2012). Several outbreaks since then have occurred, the most recent in 2012.
Hantaviruses are a heterogenous group of viruses with low and variable rates of transplacental transmission. Howard and associates (1999) reported the syndrome to cause maternal death, fetal demise, and preterm birth. They found no evidence of vertical transmission of the Sin Nombre virus. However, vertical transmission occurred inconsistently in association with hemorrhagic fever and renal syndrome caused by another Hantavirus species, the Hantaan virus.
 Enteroviruses
Enteroviruses
These viruses are a major subgroup of RNA picornaviruses that include poliovirus, coxsackievirus, and echovirus. They are trophic for intestinal epithelium but can also cause widespread maternal, fetal, and neonatal infections that may include the central nervous system, skin, heart, and lungs. Most maternal infections are subclinical yet can be fatal to the fetus-neonate (Goldenberg, 2003; Tassin, 2014). Hepatitis A is an enterovirus that is discussed in Chapter 55 (p. 1089).
Coxsackievirus
Infections with coxsackievirus group A and B are usually asymptomatic. Symptomatic infections—usually with group B—include aseptic meningitis, polio-like illness, hand foot and mouth disease, rashes, respiratory disease, pleuritis, pericarditis, and myocarditis. No treatment or vaccination is available (Cohen, 2012). Coxsackievirus may be transmitted by maternal secretions to the fetus at delivery in up to half of mothers who seroconverted during pregnancy (Modlin, 1988). Transplacental passage has also been reported (Ornoy, 2006).
Congenital malformations may be increased slightly in pregnant women who had serological evidence of coxsackievirus (Brown, 1972). Viremia can cause fetal hepatitis, skin lesions, myocarditis, and encephalomyelitis, all of which may be fatal. Koro’lkova and colleagues (1989) have also described cardiac anomalies. There is evidence of increased low-birth-weight, preterm, and small-for-gestational-age newborns (Chen, 2010). Finally, a rare association between maternal coxsackievirus infection and insulin-dependent diabetes in offspring has been reported (Dahlquist, 1996; Hyoti, 1995; Viskari, 2012).
Poliovirus
Most of these highly contagious but rare infections are subclinical or mild. The virus is trophic for the central nervous system, and it can cause paralytic poliomyelitis (Cohen, 2012). Siegel (1955) demonstrated that pregnant women not only were more susceptible to polio but also had a higher death rate. Perinatal transmission has been observed, especially when maternal infection developed in the third trimester (Bates, 1955). Inactivated subcutaneous polio vaccine is recommended for susceptible pregnant women who must travel to endemic areas or are placed in other high-risk situations. Live oral polio vaccine has been used for mass vaccination during pregnancy without harmful fetal effects (Harjulehto, 1989).
 Parvovirus
Parvovirus
Human parvovirus B19 causes erythema infectiosum, or fifth disease. The B19 virus is a small, single-stranded DNA virus that replicates in rapidly proliferating cells such as erythroblast precursors (Brown, 2012). This can lead to anemia, which is its primary fetal effect. Only individuals with the erythrocyte globoside membrane P antigen are susceptible. In women with severe hemolytic anemia—for example, sickle-cell disease—parvovirus infection may cause an aplastic crisis.
The main mode of parvovirus transmission is respiratory or hand-to-mouth contact, and the infection is common in spring months. The maternal infection rate is highest in women with school-aged children and in day-care workers but not usually in schoolteachers. Viremia develops 4 to 14 days after exposure. By adulthood, only 40 percent of women are susceptible. The annual seroconversion rate is 1 to 2 percent but is greater than 10 percent during epidemic periods (Brown, 2012). Secondary attack rates approach 50 percent.
Maternal Infection
In 20 to 30 percent of adults, infection is asymptomatic. Fever, headache, and flu-like symptoms may begin in the last few days of the viremic phase. Several days later, a bright red rash with erythroderma affects the face and gives a slapped-cheek appearance. The rash becomes lacelike and spreads to the trunk and extremities. Adults often have milder rashes and develop symmetrical polyarthralgia that may persist several weeks. There is no evidence that parvovirus infection is altered by pregnancy (Valeur-Jensen, 1999). With recovery, there is production of IgM antibody 7 to 10 days postinfection, and this persists for 3 to 4 months. Several days after IgM is produced, IgG antibody is detectable and persists for life with natural immunity.
Fetal Infection
There is vertical transmission to the fetus in up to a third of maternal parvovirus infections (Bonvicini, 2011; de Jong, 2011; Lamont, 2011a). Fetal infection has been associated with abortion, nonimmune hydrops, and stillbirth (Enders, 2010; Lassen, 2012; McClure, 2009). In a review of 1089 cases of maternal B19 infection from nine studies, Crane (2002) reported an overall fetal loss rate of 10 percent. It was 15 percent for infections before 20 weeks but was only 2.3 percent after 20 weeks. Its role in later unexplained stillbirths is unclear because most data are from retrospective cohorts with incomplete maternal and fetal histological evaluations (Norbeck, 2002; Skjöldebrand-Sparre, 2000; Tolfvenstam, 2001). Currently, there are no data to support evaluating asymptomatic mothers and stillborn fetuses for parvovirus infection.
Parvovirus is the most frequent infectious cause of nonimmune hydrops in autopsied fetuses (Rogers, 1999). That said, this complication develops only in approximately 1 percent of infected women and usually is caused by infection in the first half of gestation (Crane, 2002; Enders, 2004; Puccetti, 2012).
Yaegashi (2000) has extensively investigated the development and pathophysiology of parvovirus B19 fetal hydrops. At least 85 percent of cases of fetal infection developed within 10 weeks of maternal infection, and the mean interval was 6 to 7 weeks. More than 80 percent of hydrops cases were found in the second trimester, with a mean gestational age of 22 to 23 weeks. The critical period for maternal infection leading to fetal hydrops was estimated to be between 13 and 16 weeks—coincidental with the period in which fetal hepatic hemopoiesis is greatest.
Diagnosis and Management
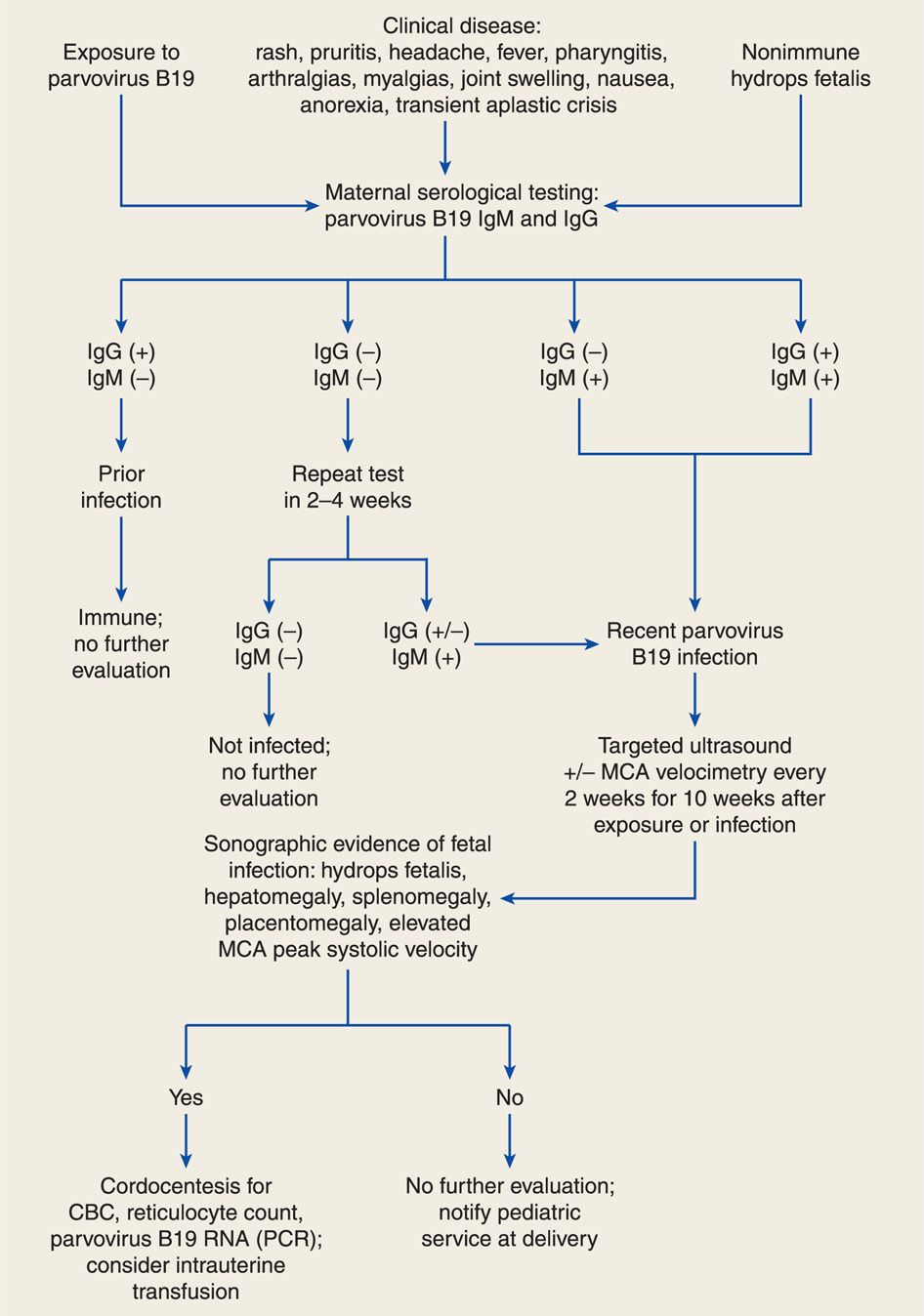
An algorithm for diagnosis of maternal parvoviral infection is illustrated in Figure 64-2. Diagnosis is generally made by maternal serological testing for specific IgG and IgM antibodies (Bonvicini, 2011; Butchko, 2004; Enders, 2006). Viral DNA may be detectable by PCR in maternal serum during the prodrome and persist for months to years after infection. Fetal infection is diagnosed by detection of B19 viral DNA in amnionic fluid or IgM antibodies in fetal serum obtained by cordocentesis (de Jong, 2011; Weiffenbach, 2012). Fetal and maternal viral loads do not predict fetal morbidity and mortality (de Haan, 2007).
FIGURE 64-2 Algorithm for evaluation and management of human parvovirus B19 infection in pregnancy. CBC = complete blood count; IgG = immunoglobulin G; IgM = immunoglobulin M; MCA = middle cerebral artery; PCR = polymerase chain reaction; RNA = ribonucleic acid.
Most cases of parvovirus-associated hydrops develop in the first 10 weeks after infection (Enders, 2004). Thus, serial sonography every 2 weeks should be performed in women with recent infection (see Fig. 64-2). Middle cerebral artery (MCA) Doppler interrogation can also be used to predict fetal anemia (Chap. 10, p. 221). Elevated peak systolic velocity values in the fetal MCA accurately predict fetal anemia (Chauvet, 2011; Cosmi, 2002; Delle Chiaie, 2001). Fetal blood sampling is warranted with hydrops to assess the degree of fetal anemia. Fetal myocarditis may induce hydrops with less severe anemia.
Depending on gestational age, fetal transfusion for hydrops may improve outcome in some cases (Enders, 2004; Schild, 1999; von Kaisenberg, 2001). Mortality rates as high as 30 percent have been reported in hydropic fetuses without transfusions. With transfusion, 94 percent of hydrops cases resolve within 6 to 12 weeks, and the overall mortality rate is < 10 percent. Most fetuses require only one transfusion because hemopoiesis resumes as infection resolves. The technique for fetal transfusion is described in Chapter 14 (p. 300).
Long-Term Prognosis
Reports describing neurodevelopmental outcomes in fetuses transfused for B19 infection-induced anemia are conflicting. Nagel and colleagues (2007) reviewed 25 transfusions in 24 hydropic fetuses. There was abnormal neurodevelopment in five of 16 survivors—32 percent—at 6 months to 8 years. Outcomes were not related to severity of fetal anemia or acidemia, and these investigators hypothesized that the infection itself induced cerebral damage. De Jong (2012) described long-term neurodevelopmental outcomes in 28 children treated with intrauterine transfusion. At a median age of 5 years, 11 percent had neurodevelopmental impairment. Conversely, Dembinski (2003) followed 20 children for a mean of 52 months after transfusion. They found no significant neurodevelopmental delay despite severe fetal anemia.
Prevention
There is currently no approved vaccine for human parvovirus B19, and there is no evidence that antiviral treatment prevents maternal or fetal infection (Broliden, 2006). Decisions to avoid higher-risk work settings are complex and require assessment of exposure risks. Pregnant women should be counseled that risks for infection approximate 5 percent for casual, infrequent contact; 20 percent for intense, prolonged work exposure such as for teachers; and 50 percent for close, frequent interaction such as in the home. Workers at day-care centers and schools need not avoid infected children because infectivity is greatest before clinical illness. Finally, infected children do not require isolation.
 Cytomegalovirus
Cytomegalovirus
This ubiquitous DNA herpes virus eventually infects most humans. Cytomegalovirus (CMV) is the most common perinatal infection in the developed world. Specifically, some evidence of fetal infection is found in 0.2 to 2.5 percent of all neonates (Kenneson, 2007). The virus is secreted into all body fluids, and person-to-person contact with viral-laden saliva, semen, urine, blood, and nasopharyngeal and cervical secretions can transmit infection. The fetus may become infected by transplacental viremia, or the neonate is infected at delivery or during breast feeding. Moreover, acquisition continues to accrue. Day-care centers, for example, are a frequent source, and by 2 to 3 years of age, many children have infected one another and may transmit infection to their parents (Demmler, 1991; Pass, 1991). Revello and coworkers (2008) reported that amniocentesis in women whose blood is positive for CMV DNA does not result in iatrogenic fetal transmission.
Up to 85 percent of women from lower socioeconomic backgrounds are seropositive by the time of pregnancy, whereas only half of women in higher income groups are immune. Following primary CMV infection, and in a manner similar to other herpes virus infections, the virus becomes latent with periodic reactivation characterized by viral shedding. This occurs despite high serum levels of anti-CMV IgG antibody. These antibodies do not prevent maternal recurrence, reactivation, or reinfection, nor do they totally mitigate fetal or neonatal infection.
Women who are seronegative before pregnancy but who develop primary CMV infection during pregnancy are at greatest risk to have an infected fetus. It is estimated that 25 percent of congenital CMV infections in the United States are from primary maternal infection (Wang, 2011). Because most CMV infections are clinically silent, they are detected by seroconversion, and this may be as high as 1 to 7 percent (Hyde, 2010).
Maternal Infection
Pregnancy does not increase the risk or severity of maternal CMV infection. Most infections are asymptomatic, but 10 to 15 percent of infected adults have a mononucleosis-like syndrome characterized by fever, pharyngitis, lymphadenopathy, and polyarthritis. Immunocompromised women may develop myocarditis, pneumonitis, hepatitis, retinitis, gastroenteritis, or meningoencephalitis. Nigro and associates (2003) reported that most women in a cohort with primary infection had elevated serum aminotransferases or lymphocytosis. Reactivation disease usually is asymptomatic, although viral shedding is common.
Primary maternal CMV infection is transmitted to the fetus in approximately 40 percent of cases and can cause severe morbidity (Fowler, 1992; Liesnard, 2000). In contrast, recurrent maternal infection infects the fetus in only 0.15 to 1 percent of cases. A review of nine studies of CMV vertical transmission rates reported first-trimester transmission in 36 percent, second-trimester in 40 percent, and third-trimester in 65 percent (Picone, 2013). Naturally acquired immunity during pregnancy results in a 70-percent risk reduction of congenital CMV infection in future pregnancies (Fowler, 2003). However, as noted earlier, maternal immunity does not prevent recurrences, and maternal antibodies do not prevent fetal infection. Also, some seropositive women can be reinfected with a different viral strain that can cause fetal infection and symptomatic congenital disease (Ross, 2011).
Fetal Infection
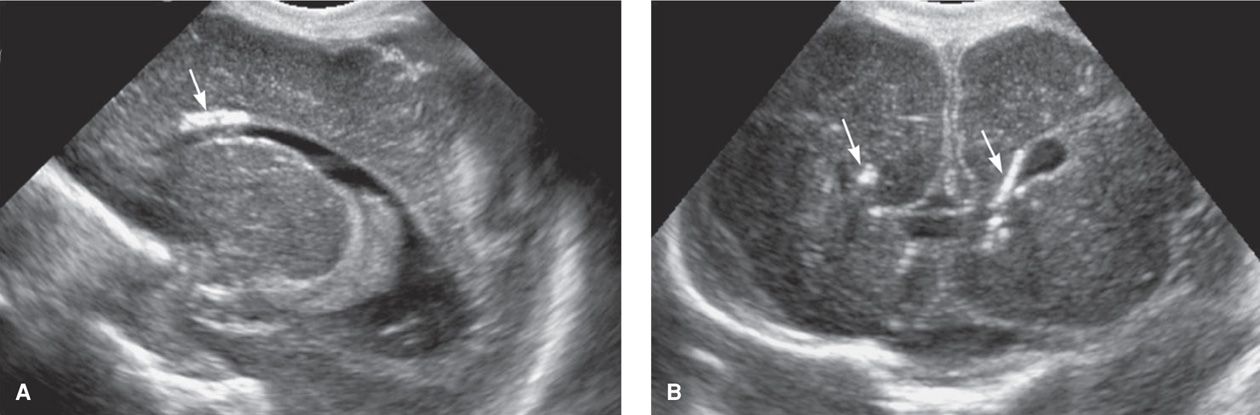
When a newborn has apparent sequelae of in utero-acquired CMV infection, it is referred to as symptomatic CMV infection. Congenital infection is a syndrome that may include growth restriction, microcephaly, intracranial calcifications, chorioretinitis, mental and motor retardation, sensorineural deficits, hepatosplenomegaly, jaundice, hemolytic anemia, and thrombocytopenic purpura (Fig. 64-3). The pathogenesis of these outcomes has been reviewed by Cheeran and colleagues (2009). Of the estimated 40,000 infected neonates born each year, only 5 to 10 percent demonstrate this syndrome (Fowler, 1992). Thus, most infected infants are asymptomatic at birth, but some develop late-onset sequelae. They may include hearing loss, neurological deficits, chorioretinitis, psychomotor retardation, and learning disabilities.
FIGURE 64-3 Sagittal (A) and coronal (B) cranial sonograms from a neonate with congenital cytomegalovirus infection. The arrows indicate periventricular calcifications.
Prenatal Diagnosis
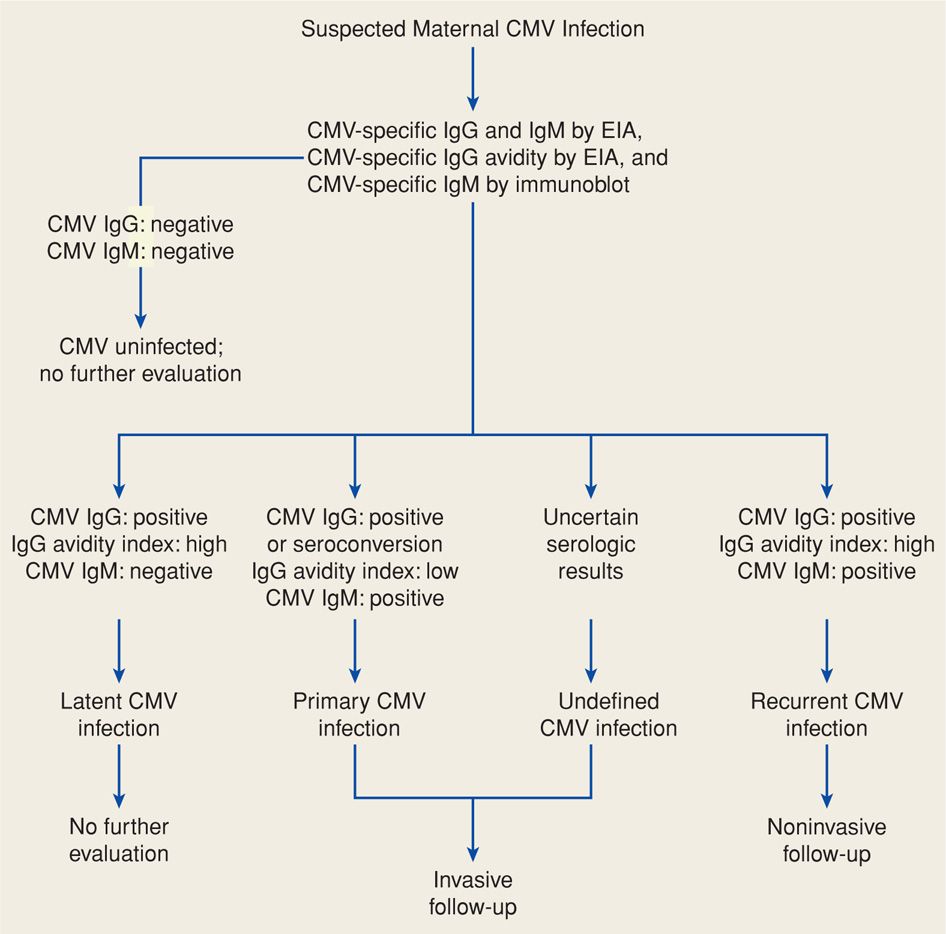
Routine prenatal CMV serological screening is currently not recommended. Pregnant women should be tested for CMV if they present with a mononucleosis-like illness or if congenital infection is suspected based on abnormal sonographic findings. Primary infection is diagnosed using CMV-specific IgG testing of paired acute and convalescent sera. CMV IgM does not accurately reflect timing of seroconversion because IgM antibody levels may be elevated for more than a year (Stagno, 1985). Moreover, CMV IgM may be found with reactivation disease or reinfection with a new strain. Thus, specific CMV IgG avidity testing is valuable in confirming primary CMV infection (Fig. 64-4). High anti-CMV IgG avidity indicates primary maternal infection > 6 months before testing (Kanengisser-Pines, 2009). Finally, viral culture may be useful, although a minimum of 21 days is required before culture findings are considered negative.
FIGURE 64-4 Algorithm for evaluation of suspected maternal primary cytomegalovirus (CMV) infection in pregnancy. EIA = enzyme immunoassay; IgG = immunoglobulin G; IgM = immunoglobulin M.
Several fetal abnormalities associated with CMV infection may be seen with sonography, computed tomography, or magnetic resonance imaging. In some cases, they are found at the time of routine prenatal sonographic screening, but in others they are part of a specific evaluation in women with CMV infection. Findings include microcephaly, ventriculomegaly, and cerebral calcifications; ascites, hepatomegaly, splenomegaly, and hyperechoic bowel; hydrops; and oligohydramnios (Malinger, 2003). Abnormal sonographic findings seen in combination with positive findings in fetal blood or amnionic fluid are predictive of an approximate 75-percent risk of symptomatic congenital infection (Enders, 2001).
CMV nucleic acid amplification testing of amnionic fluid is considered the gold standard for the diagnosis of fetal infection. Sensitivities range from 70 to 99 percent and depend on amniocentesis timing (Nigro, 2005; Revello, 2004). Sensitivity is highest when amniocentesis is performed at least 6 weeks after maternal infection and after 21 weeks (Azam, 2001; Guerra, 2000). A negative amnionic fluid PCR does not exclude fetal infection and may need to be repeated if suspicion for fetal infection is high.
Management and Prevention
The management of the immunocompetent pregnant woman with primary or recurrent CMV is limited to symptomatic treatment. If recent primary CMV infection is confirmed, amnionic fluid analysis should be offered. Counseling regarding fetal outcome depends on the gestation age during which primary infection is documented. Even with the high infection rate with primary infection in the first half of pregnancy, most fetuses develop normally. However, pregnancy termination may be an option for some.
Kimberlin (2003) showed that intravenous ganciclovir administered for 6 weeks to neonates with symptomatic central nervous system disease prevents hearing deterioration at 6 months and possibly later. Conversely, antiviral chemotherapy given antepartum does not avert in utero CMV transmission. Passive immunization with CMV-specific hyperimmune globulin lowers the risk of congenital CMV infection when given to pregnant women with primary disease (Nigro, 2005, 2012; Visentin, 2012). Further clinical trials are necessary before this becomes standard treatment (McCarthy, 2011).
There is no CMV vaccine. Prevention of congenital infection relies on avoiding maternal primary infection, especially in early pregnancy. Basic measures such as good hygiene and hand washing have been promoted, particularly for women with toddlers in day-care settings (Fowler, 2000). Although there may be sexual transmission from infected partners, there are no data on the efficacy of preventive strategies.
BACTERIAL INFECTIONS
 Group A Streptococcus
Group A Streptococcus
Infections caused by Streptococcus pyogenes are important in pregnant women. It is the most frequent bacterial cause of acute pharyngitis and is associated with several systemic and cutaneous infections. S pyogenes produces numerous toxins and enzymes responsible for the local and systemic toxicity associated with this organism. Pyrogenic exotoxin-producing strains are usually associated with severe disease (Mason, 2012; Wessels, 2012). In most cases, streptococcal pharyngitis, scarlet fever, and erysipelas are not life threatening. Treatment, usually with penicillin, is similar in pregnant and nonpregnant women (Shulman, 2012).
In the United States, Streptococcus pyogenes infrequently causes puerperal infection. Still, it remains the most common cause of severe maternal postpartum infection and death worldwide, and the incidence of these infections is increasing (Deutscher, 2011; Hamilton, 2013; Mason, 2012; Wessels, 2012). Puerperal infections are discussed in detail in Chapter 37 (p. 682). The early 1990s saw the rise of streptococcal toxic shock syndrome, manifested by hypotension, fever, and evidence of multiorgan failure with associated bacteremia. The case-fatality rate approximates 30 percent, and morbidity and mortality rates are improved with early recognition. Treatment includes clindamycin or penicillin therapy and often surgical debridement (Hamilton, 2013). No vaccine for group A streptococcus is commercially available.
 Group B Streptococcus
Group B Streptococcus
Streptococcus agalactiae is a group B organism that can be found to colonize the gastrointestinal and genitourinary tract in 20 to 30 percent of pregnant women. Throughout pregnancy, group B Streptococcus (GBS) is isolated in a transient, intermittent, or chronic fashion. Although the organism is most likely always present in these same women, their isolation is not always homologous.
Maternal and Perinatal Infection
The spectrum of maternal and fetal GBS ranges from asymptomatic colonization to septicemia. Streptococcus agalactiae has been implicated in adverse pregnancy outcomes, including preterm labor, prematurely ruptured membranes, clinical and subclinical chorioamnionitis, and fetal infections. GBS can also cause maternal bacteriuria, pyelonephritis, osteomyelitis, postpartum mastitis, and puerperal infections. It remains the leading infectious cause of morbidity and mortality among infants in the United States (Centers for Disease Control and Prevention, 2010; Schrag, 2003; Wessels, 2012).
Neonatal sepsis has received the most attention due to its devastating consequences and available effective preventative measures. Infection < 7 days after birth is defined as early-onset disease and is seen in 0.24/1000 live births (Centers for Disease Control and Prevention, 2013a). Many investigators use a threshold of < 72 hours of life as most compatible with intrapartum acquisition of disease (Pulver, 2009; Wendel, 2002). We and others have also encountered several unexpected intrapartum stillbirths from GBS infections. Tudela and associates (2012) recently reported that newborns with early-onset GBS infection often had clinical evidence of fetal infection during labor or at delivery.
In many neonates, septicemia involves signs of serious illness that usually develop within 6 to 12 hours of birth. These include respiratory distress, apnea, and hypotension. At the outset, therefore, neonatal infection must be differentiated from respiratory distress syndrome caused by insufficient surfactant production of the preterm neonate (Chap. 34, p. 653). The mortality rate with early-onset disease has declined to approximately 4 percent, and preterm newborns are disparately affected.
Late-onset disease caused by GBS is noted in 0.32 per 1000 live births and usually manifests as meningitis 1 week to 3 months after birth (Centers for Disease Control and Prevention, 2013a). The mortality rate, although appreciable, is less for late-onset meningitis than for early-onset sepsis. Unfortunately, it is not uncommon for surviving infants of both early- and late-onset disease to exhibit devastating neurological sequelae.
Prophylaxis for Perinatal Infections
As GBS neonatal infections evolved beginning in the 1970s and before widespread intrapartum chemoprophylaxis, rates of early-onset sepsis ranged from 2 to 3 per 1000 live births. In 2002, the Centers for Disease Control and Prevention, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics revised guidelines for perinatal prevention of GBS disease. They recommended universal rectovaginal culture screening for GBS at 35 to 37 weeks’ gestation followed by intrapartum antibiotic prophylaxis for women identified to be carriers. Subsequent to implementation of these guidelines, the incidence of early-onset GBS neonatal sepsis has decreased to 0.24 cases per 1000 live births by 2012 (Centers for Disease Control and Prevention, 2013a). These guidelines were updated for early-onset GBS infection in 2010. They expanded laboratory identification criteria for GBS; updated algorithms for screening and intrapartum chemoprophylaxis for women with preterm prematurely ruptured membranes, preterm labor, or penicillin allergy; and described new dosing for penicillin G chemoprophylaxis.
Thus, during the past three decades, several strategies have been proposed to prevent perinatal acquisition of GBS infections (Ohlsson, 2013). These strategies have not been compared in randomized trials and are either culture-based or risk-based guidelines as subsequently discussed.
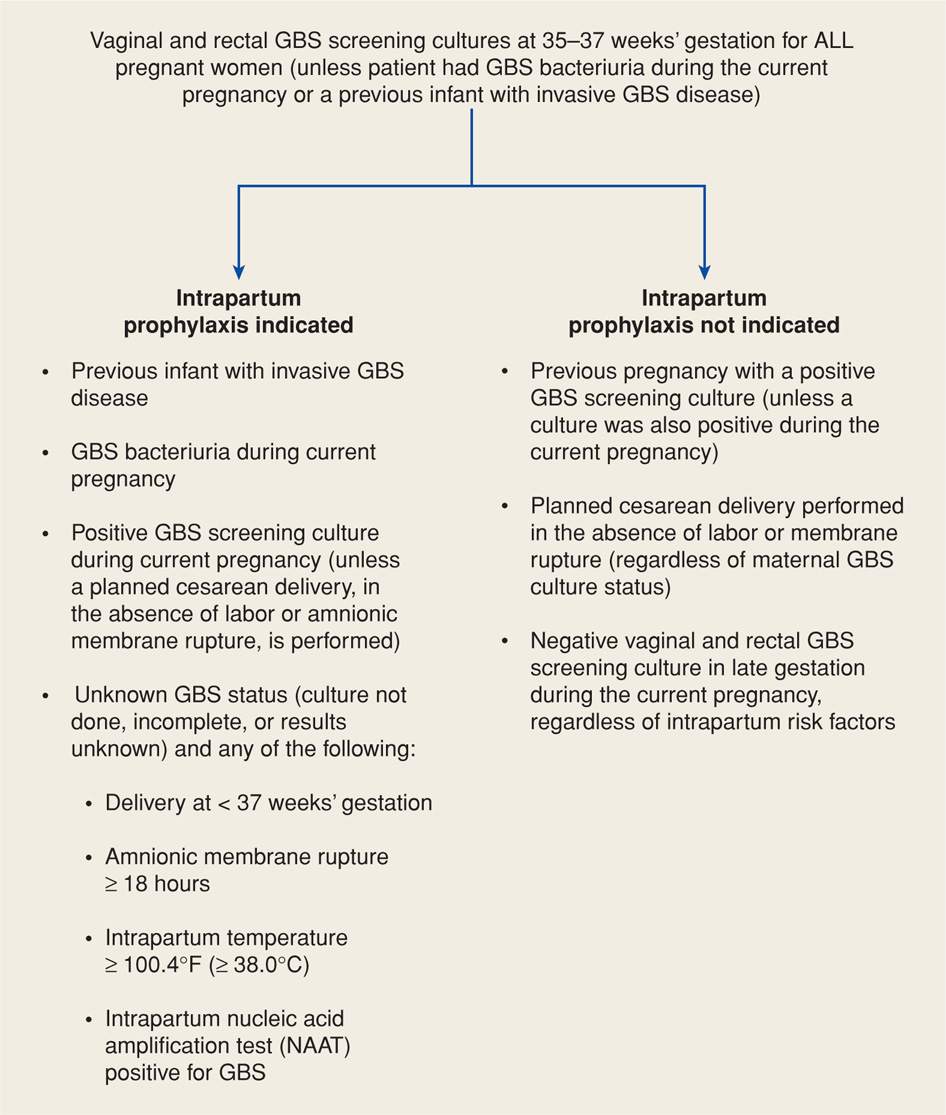
Culture-Based Prevention. The 2010 Centers for Disease Control and Prevention GBS Guidelines recommend a culture-based approach as shown in Figure 64-5. Such a protocol was also adopted by the American College of Obstetricians and Gynecologists (2013c). This approach is designed to identify women who should be given intrapartum antimicrobial prophylaxis. Women are screened for GBS colonization at 35 to 37 weeks’ gestation, and intrapartum antimicrobials are given to women with rectovaginal GBS-positive cultures. Selective enrichment broth followed by subculture improves detection. In addition, more rapid techniques such as DNA probes and nucleic acid amplification tests are being developed (Chan, 2006; Helali, 2012). A previous sibling with GBS invasive disease and identification of GBS bacteriuria in the current pregnancy are also considered indications for prophylaxis.
FIGURE 64-5 Indications for intrapartum prophylaxis to prevent perinatal group B streptococcal (GBS) disease under a universal prenatal screening strategy based on combined vaginal and rectal cultures obtained at 35 to 37 weeks’ gestation. (From Centers for Disease Control and Prevention, 2010.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree