Acute otitis media (AOM) results in fluid accumulation behind the middle ear with subsequent inflammation.
One of the most common infections for which children are prescribed antibiotics; some estimate over 12 million prescriptions annually.
Most commonly seen in conjunction with viral respiratory tract infections, including respiratory syncytial virus (RSV), parainfluenza, influenza, rhinovirus, or adenovirus
Most cases of AOM are felt to be viral without the presence of bacteria.
Common bacterial pathogens include Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, group A and B streptococcus, and rarely
In classic cases, children will present with fever, ear pain, and/or decreased hearing.
Younger infants may present with nonspecific complaints including ear tugging, malaise, vomiting, congestion, cough, or fussiness.
AOM must be differentiated from otitis media with effusion (OME), since OME does not warrant antimicrobial therapy.
Essential to the diagnosis is otoscopy. The best and most reproducible finding of AOM is bulging of the tympanic membrane (TM). Other findings that are less specific are retraction, opacification, erythema, and decreased mobility of the TM. Presence of purulent-appearing fluid or air bubbles may also be helpful.
OME is typified by the presence of a middle ear effusion without signs of inflammation, as these TMs are not bulging or erythematous.
Pain control is an essential part of therapy, as NSAIDs and acetaminophen provide symptomatic relief.
The 2013 American Academy of Pediatrics and the American Academy of Family Physicians produced a guideline of treatment for AOM.
In the recommendations, children under age 6 months should be treated for AOM with antibiotics, whereas for children older than 6 months with nonsevere (unilateral, mild otalgia, otalgia for <48 hours, or temperature below 39°C) AOM, a period of watchful observation may be offered. For children 6 months to 23 months with bilateral AOM, even with mild symptoms, treatment should be offered. Meta-analyses show that treatment of AOM with antibiotics only results in modest improvement in symptoms and that many cases are self-resolving and/or caused by viral pathogens.
If antibiotic therapy is to be initiated, first-line antibiotic therapy should be amoxicillin 90 mg/kg/day divided twice a day. If a child fails to improve, next-line
therapy is amoxicillin/clavulanic acid at 90 mg/kg/day (of the amoxicillin component) divided twice a day. The rationale behind this choice is that the pathogens may have a beta lactamase-conferring resistance to amoxicillin, but the usage of clavulanic acid may restore susceptibility.
If the infection continues, consider administration of IM ceftriaxone for improved activity against potentially resistant pathogens. ENT referral for tympanocentesis may be indicated.
If a child has multiple episodes of AOM, at least 3 episodes in 6 months or 4 episodes in 1 year, this may be an indication for referral to ENT for myringotomy tube placement. If there are other infections or poor weight gain, an underlying immunodeficiency may also be considered.
One of the most common causes of pediatric hospitalization in an otherwise healthy host
Typically afflicts children under the age of 2 years, with a peak incidence between ages 2 and 6 months
Caused by viral infection, most common agents include RSV, human metapneumovirus, parainfluenza, rhinovirus, influenza, adenoviruses, and coronaviruses. Also associated with Bordetella pertussis and Mycoplasma pneumoniae.
Incidence corresponds to peaks in viral activity, predominantly during winter months, although this condition can be seen during all times of the year.
Initial symptoms include congestion and nasal discharge. Fever may also be observed:
Progression of the disease involves lower airways, leading to cough, tachypnea, and respiratory distress.
Clinical exam can be quite variable but is typically associated with diffuse crackles or wheezes, associated with signs of respiratory distress including nasal flaring, grunting, and retractions.
Hypoxemia is a common indication for hospitalization.
Clinical course is variable, but younger infants, history of prematurity, immunodeficiency, or chronic lung disease may lead to prolonged duration of illness.
Bronchiolitis is a clinical diagnosis; additional testing is not required.
Blood gases may be helpful in determining which infants may require more intensive respiratory interventions.
Chest x-ray (CXR) commonly shows atelectasis, hyperexpansion, or diffuse peribronchiolar infiltrates.
Atelectasis arising from viral bronchiolitis can be confused with lobar consolidation seen with bacterial pneumonia.
Viral testing is not commonly recommended, although it may be beneficial for infection control and patient cohorting. Testing for influenza may identify infants who would be a candidate for oseltamivir treatment and prophylaxis of family members.
Current recommendations are supportive care, including oxygen as needed, nasal suctioning, and hydration.
Bronchodilator therapy is controversial, but there may be benefit on a case-by-case basis on usage of albuterol or inhaled epinephrine. Other interventions such as nebulized hypertonic saline may lead to potential benefit, but its impact is variable.
There is no clear evidence of significant benefit of antivirals or steroids with bronchiolitis. Identification of infants with influenza may benefit from oseltamivir.
More severe infections may require respiratory interventions like CPAP, mechanical ventilation, or rarely ECMO.
Focal findings on chest auscultation or persistence of symptoms beyond the expected duration of illness should prompt consideration for treatment of pneumonia.
Palivizumab (Synagis) is available for prophylaxis of RSV infection in selected infants; see Table 20-1.
Bronchiolitis is associated with future reactive airway disease (RAD), but it is unclear if this association is due to bronchiolitis increasing the risk of RAD or if infants who have underlying risks for RAD are at increased risk for developing bronchiolitis.
TABLE 20-1 Palivizumab Prophylaxis for Respiratory Syncytial Virus (Including 2014 Update) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Single greatest cause of pediatric death worldwide
Increasing data suggest viral pathogens are the most common cause of pneumonia, up to 80% of community-acquired pneumonia in children under the age of two. Agents include RSV, parainfluenza, influenza, human metapneumoviruses, adenoviruses, and rhinoviruses.
Common bacterial pathogens include S. pneumoniae, H. influenzae, S. aureus, M. pneumoniae, and B. pertussis. In newborns, other pathogens must be considered including group B streptococcus, enteric Gram negatives, Chlamydia trachomatis, or Treponema pallidum.
Depending on the host, immunosuppression, and exposure history, additional infectious agents include Chlamydophila pneumoniae, Chlamydophila psittaci, Legionella pneumophila, Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis, Cryptococcus species, Francisella tularensis, cytomegalovirus (CMV), herpes simplex virus (HSV), or Mycobacterium sp. (including M. tuberculosis).
There is a rising incidence of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia that commonly is associated with severe necrotizing disease.
Children typically present with fever, cough, and tachypnea, less commonly with fatigue, chest pain, or abdominal pain.
Physical examination typically reveals focal findings of decreased breath sounds, wheezing, crackles, or egophony.
Pulse oximetry should be performed in all children with clinical suspicion of pneumonia.
Viral testing may aid in the determination to treat with antibiotics or therapy against influenza, but there is a significant false-positive rate, and cases of viral infection with bacterial superinfection.
Additional testing is available for some of other etiologies of pneumonia, including serology, antigen testing, or polymerase chain reaction (PCR).
CXR will routinely show lobar consolidation with a typical bacterial pneumonia. With MRSA pneumonia, lung abscess or necrotizing pneumonia may also be seen.
The Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) do not recommend routine CXR for patients who that will be managed in the outpatient setting. Studies have shown CXR findings do not routinely change clinical care in this scenario. CXR should be performed in children being hospitalized or in evaluating children who have not responded to therapy to evaluate for effusion or empyema formation.
Classically M. pneumoniae appears as a diffuse pneumonia on CXR; lobar consolidation can be observed.
Blood cultures are infrequently positive (1%-8.2%).
In preschool children with mild disease and close follow-up, both IDSA and PIDS recommend against routine antimicrobials as it is mostly like a viral etiology, and that only supportive care is necessary.
If antimicrobial treatment is to be initiated, first-line recommendations are amoxicillin (90 mg/kg/day divided BID) or ampicillin (150-200 mg/kg/day divided q6h).
Azithromycin should be considered for a high concern for Mycoplasma or Chlamydophila pneumonia.
Additional therapy with 3rd-generation cephalosporins, clindamycin, or vancomycin may be considered for severe pneumonia or for children who fail to respond to initial therapy.
Most treatment regimens are 10-14 days in duration, longer for complicated pneumonia.
Recurrent pneumonias should be a prompt for an evaluation of immunodeficiency, cystic fibrosis, ciliary dyskinesia, or structural defect.
Urinary tract infection (UTI) is the most common cause of renal parenchymal damage.
During the 1st year of life, males are affected more than are females; but after the 1st year, females are more likely to develop a UTI.
Most common bacterial pathogens include Escherichia coli, other Gram-negative bacteria (e.g., Klebsiella and Proteus), enterococci, Staphylococcus saprophyticus, and group B streptococcus.
Typical symptoms are dysuria, abdominal pain, malodorous urine, and fever.
Less common symptoms include nausea, vomiting, or fussiness.
Diagnosis requires the presence of both: (1) pyuria and (2) isolation of a bacterial pathogen in sufficient quantity. The lack of one of these factors would suggest against a diagnosis of a UTI.
Finding pyuria on urinalysis is based on the presence of >5 white blood cells (WBCs)/high-power field. If urine microscopy is not available, presence of leukocyte esterase can be substituted.
Urinary nitrites are seen with only certain pathogens (Gram-negative organisms) and if the urine has had sufficient dwell time in the urinary bladder. In younger infants who do not have a long urinary dwell time, nitrites are commonly not detected even when a Gram-negative pathogen is present. Positive urinary nitrites have high specificity for UTI.
Significant urinary culture results depend on the source of sample. Thresholds of >50,000 or >100,000 colony-forming units (CFUs)/mL have been utilized for catheterized or clean catch samples, while isolation of any bacteria from a suprapubic tap is considered significant culture growth.
Bagged urinary specimens are prone to contamination from perineal organisms and should not be routinely used for establishing a diagnosis of a UTI. However, a negative bagged specimen does eliminate the possibility of a UTI.
If a urine sample has a positive culture result, but no evidence of pyuria, this may be reflective of three possibilities: (1) early UTI without a significant inflammatory response, (2) asymptomatic bacteriuria, or (3) contamination of the sample. A repeat sample should be obtained >24 hours later, even if the child is on antibiotics, as presence of pyuria would indicated that the previous sample was indeed consistent with an early UTI. If pyuria remains absent, this would suggest asymptomatic bacteriuria or contamination, and neither condition would require treatment.
Ultrasound should be considered in febrile infants or in children with recurrent UTIs.
A voiding cystourethrogram (VCUG) should be considered in children with abnormalities found on ultrasound or in those with recurrent episodes of febrile UTIs. VCUG should not be completed during the acute phase of the UTI.
Treatment can be guided toward common urinary pathogens, as antimicrobials such as amoxicillin, cefdinir, ceftriaxone, trimethoprim-sulfamethoxazole, and nitrofurantoin all provide excellent empiric coverage as culture results are finalized. Nitrofurantoin should be avoided in pyelonephritis. Treatment duration is between 7 and 14 days.
For some children with structural abnormalities, daily prophylaxis can be considered with trimethoprim-sulfamethoxazole, nitrofurantoin, or amoxicillin. The usage of prophylaxis in urinary reflux is controversial, with some evidence demonstrating no benefit and other studies indicating lower incidence of UTI but increased rates of antibiotic resistance among urinary pathogens.
Children with structural abnormalities found on ultrasound should be referred to a urologist.
At least 1%-3% of children develops asymptomatic carriage of bacteria in their urinary tract, which is not associated with future development of UTIs or renal scarring, and should not be routinely treated with antibiotics.
Fever in infants <90 days of life is defined as a rectal temperature equal to or >38°C.
Infants with fever present a challenge and deserve special consideration. Infants under 90 days of life lack a fully developed immune system, are exposed to a unique group of bacterial pathogens, and often do not localize a source of infection.
Because of these risk factors, infants are at significant risk for serious bacterial infections (SBIs), including UTI, bacteremia, meningitis, pneumonia, and skin/soft tissue infection. In one study, up to 13.5% of febrile infants had an identified SBI. UTIs are the most frequently identified infection, accounting for up to 92% of all cases of SBI. Meningitis accounts for around 1% of all febrile cases, with a higher risk for infants under 30 days of life.
Many studies have evaluated screening tools to identify febrile infants most likely to have an SBI. One approach used by several centers is performing blood, urine, and cerebrospinal fluid (CSF) studies on all infants under 60 days of life, regardless of physical examination and laboratory results. There are cases of infants with normal physical examination and initial laboratory studies who are found to have meningitis. Alternatively, some centers make a clinical decision regarding performing a lumbar puncture for infants 30 to 90 days of life. See Table 20-2, which presents one institution’s approach to the febrile infant. Remember that although guidelines and flow charts may be useful in managing certain classes of patients, diagnostic testing and management decisions should always incorporate clinical judgment.
Although some clinicians would obtain a chest radiograph for all febrile infants, others consider this examination only in infants with signs of respiratory distress including tachypnea, nasal flaring, retractions, grunting, crackles, rhonchi, wheezing, cough, or rhinitis.
Clinical bronchiolitis or positive testing for RSV or influenza significantly reduces the risk of SBIs. In several medium-sized trials, there have been no cases of meningitis with infants who have bronchiolitis or are positive for RSV or influenza, but there are isolated case reports of meningitis with bronchiolitis. The incidence of UTI and bacteremia is also reduced, but still at significant incidence.
Indications for HSV testing and empiric acyclovir therapy in neonates with fever:
There are no published criteria to apply in deciding which febrile neonates should be evaluated and treated empirically for HSV infection. Decisions may be guided by physical findings (e.g., skin lesions), presenting symptoms (e.g., lethargy or seizures), or local practice patterns.
Furthermore, there have been case reports of neonates with HSV meningitis who lack a CSF pleocytosis; thus, the lack of CSF pleocytosis cannot be used to rule out the possibility of HSV disease.
Consultation with an infectious diseases specialist may be warranted.
TABLE 20-2 Approach to the Febrile Neonate | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Young infants may present only with fever or temperature instability, irritability, somnolence, poor feeding, vomiting, and seizures.
Older children may experience fever, headache, neck pain or stiffness, nausea and vomiting, photophobia, and irritability.
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) occurs in 30%-60% of children with bacterial meningitis.
In infants, examination may reveal a bulging fontanelle.
Common physical findings include lethargy, somnolence, meningismus, rash (including petechiae and/or purpura), and hemodynamic instability. Kernig and Brudzinski signs may be found in older children, but not typically in infants.
Seizures can occur in 20%-30% of patients within the first 3 days of their meningitis course, usually resulting from inflammation. However, seizures are more common with encephalitis. In many children, fever may persist for 5 days after initiation of appropriate antibiotic therapy.
The diagnosis is made on the basis of CSF findings after LP. CSF findings in meningitis are presented in Table 20-3.
In the event of a traumatic LP, some clinicians use a correction factor to help discern which patients are unlikely to have meningitis and thus do not need to be admitted to the hospital.
A recent study found that a CSF WBC:red blood cell (RBC) ratio of ≤1:100 (0.01) and an observed-to-predicted CSF WBC count ratio of ≤0.01 have a high positive
predictive value for predicting the absence of meningitis, where CSF predicted WBC count = CSF RBC × (peripheral blood WBC/peripheral blood RBC).
However, such ratios must be interpreted in the context of other parameters, including the CSF WBC differential, glucose, and Gram stain, as well as the patient’s clinical appearance and whether the patient was pretreated with antibiotics.
TABLE 20-3 Cerebrospinal Fluid Parameters in Suspected Meningitis | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Give empiric antibiotic therapy as presented in Table 20-4.
Duration of therapy varies with etiology, as shown in Table 20-5.
Corticosteroids have been administered to patients with bacterial meningitis with the purpose of decreasing inflammation and thus decreasing the risk of hearing loss. However, conflicting literature exists regarding the benefit of corticosteroids in improving neurologic sequelae or reducing hearing loss.
Current American Academy of Pediatrics (AAP) guidelines state that dexamethasone should be recommended in conjunction with antibiotics for children with H. influenzae type b meningitis. The AAP guidelines state that dexamethasone therapy should be considered for infants and children with pneumococcal meningitis who are at least 6 weeks of age.
If dexamethasone is used, it should be given prior to or concurrently with the first antibiotic dose.
TABLE 20-4 Common Etiologies and Empiric Antibiotics for Meningitis | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
TABLE 20-5 Duration of Antibiotic Therapy Based for Children with Meningitisa | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Considerations for repeat lumbar puncture include the following:
Meningitis caused by resistant strains of S. pneumoniae
Meningitis caused by Gram-negative bacilli
Lack of clinical improvement 24-36 hours after the start of therapy
Prolonged (>5 days) or secondary fever
Recurrent meningitis
Immunocompromised host
All children with bacterial meningitis require a hearing evaluation. Sensorineural hearing loss occurs in ˜30% of children with pneumococcal meningitis and in 5%-10% of children with meningococcal and H. influenzae meningitis.
Signs and symptoms include fever, seizures, altered mental status, personality changes, and focal neurologic findings.
Onset is acute.
Untreated disease progresses to coma and death.
CSF reveals elevated WBC (25-1,000/mm3) with a predominance of lymphocytes.
Erythrocytes are present in the CSF in 50% of cases.
HSV (usually HSV-1) can be detected in CSF by PCR.
Electroencephalography may reveal a specific pattern of periodic lateralizing epileptiform discharges (PLEDs).
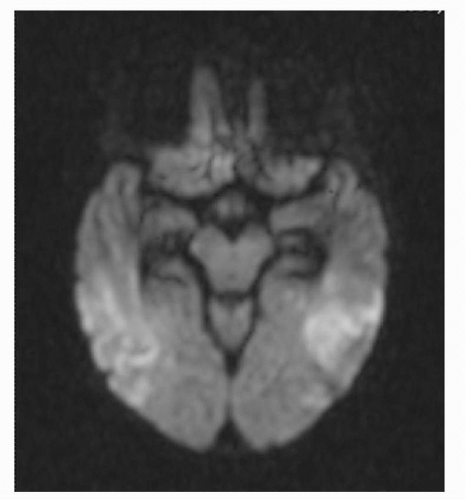
Magnetic resonance imaging (MRI) is significantly more sensitive than is computed tomography in HSV encephalitis. Typical MRI findings include abnormal edema or hemorrhagic necrosis involving the white matter of the temporal lobe region (Fig. 20-1), though involvement in children with HSV-1 encephalitis may be more multifocal.
IV acyclovir should be given 60 mg/kg/day divided every 8 hours, typically for 21 days.
Infectious mononucleosis is most commonly caused by Epstein-Barr virus (EBV) and is transmitted via close personal contact or sharing of eating and drinking utensils.
Other causes of infectious mononucleosis-like illness include CMV, toxoplasmosis, human immunodeficiency virus (HIV), rubella, hepatitis A virus (HAV), human herpesvirus 6 (HHV-6), and adenovirus.
Signs and symptoms include fever, exudative pharyngitis, headache, generalized lymphadenopathy, malaise, and hepatosplenomegaly. A morbilliform rash may occur in patients with EBV infection who are treated with penicillin antibiotics, especially ampicillin.
Symptoms typically last 1 week to 1 month in duration, and fatigue may persist for several months.
Unusual complications include central nervous system (CNS) manifestations (aseptic meningitis, encephalitis, Guillain-Barré syndrome, cranial or peripheral neuropathies), splenic rupture, thrombocytopenia, agranulocytosis, hemolytic anemia, hemophagocytic syndrome, orchitis, and myocarditis.
TABLE 20-6 Serum Epstein-Barr Virus (EBV) Antibodies in EBV Infection | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Although the heterophile antibody test (Monospot) is often negative in children <4 years of age, it can identify 90%-98% of cases in older children and adults.
Diagnosis also may be made via EBV antibody tests, including IgM and IgG to the viral capsid antigen (VCA), antibody to the early antigen (EA) complex diffuse component, and antibody to the EBV-associated nuclear antigen (EBNA).
All antibody tests may be negative in patients presenting in their first days of illness.
EBV DNA can often be detected by PCR in the blood during acute mononucleosis, but this testing is not recommended in the evaluation of routine cases. Viral reactivation during other illnesses is a frequent occurrence.
Table 20-6 presents information about the interpretation of EBV antibodies in infectious mononucleosis.
Patients with active infection may exhibit elevated serum transaminases.
A rise in the proportion of atypical lymphocytes in the peripheral smear, often >10%, usually occurs during the 2nd week of illness. However, this finding is less common in young children.
Supportive care is appropriate.
Corticosteroids may be used in patients with marked tonsillar inflammation with impending airway obstruction, massive splenomegaly, myocarditis, hemolytic anemia, aplastic anemia, hemophagocytic syndrome, or neurologic disease.
Patients should avoid contact sports until fully recovered and the spleen is no longer palpable (typically > 6 weeks).
For further information, see Table 20-7.
TABLE 20-7 The Numbered Exanthems of Childhood | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
A benign, self-limited entity consisting of acute, fixed, erythematous macules that develop into papules and target lesions in which the central portion of the lesion becomes dusky or necrotic surrounded by concentric rings of erythema. These target lesions may coalesce to form plaques.
In many cases, a definite cause is not identified. The most common infectious causes are HSV, M. pneumoniae, and group A Streptococcus.
The initial surrounding blanching erythema may resemble hives or insect bites. Lesions in different stages can be seen at the same time. With resolution of the lesions, scaling, desquamation, hyperpigmentation, or hypopigmentation may occur.
The rash is usually symmetric and involves the hands, mouth, face, palms, soles, and extensor surfaces of the extremities. It may also affect the conjunctiva, genital tract, or upper airway.
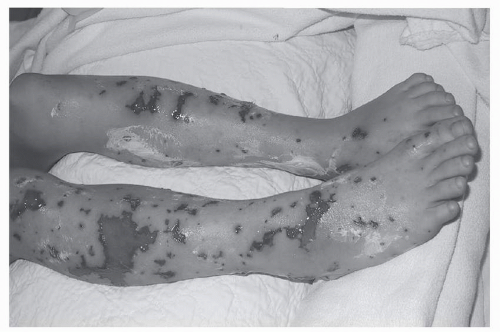
Petechial rashes necessitate prompt evaluation to exclude severe, life-threatening illness.
The most common infectious causes of petechiae are:
Meningococcemia (Neisseria meningitidis) (Fig. 20-3):
Prodrome: cough, headache, sore throat, nausea, and vomiting
Acute illness: petechial rash, high spiking fevers, tachypnea, tachycardia, and hypotension
Other bacterial causes: Rickettsia rickettsii (Rocky Mountain spotted fever) (Fig. 20-4), Rickettsia prowazekii (endemic typhus), N. gonorrhoeae, Pseudomonas aeruginosa, Streptococcus pyogenes, and Capnocytophaga canimorsus
Viral causes: enteroviruses (especially coxsackievirus A4, A9, and B2-B5 and echovirus 3, 4, 7, 9, and 18), EBV, CMV, parvovirus B19, hepatitis virus B and C, rubeola virus (typical and atypical measles), and viral hemorrhagic fevers caused by arboviruses and arenaviruses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree