Table 43–2. Some parasitic infections rarely seen in developed regions.
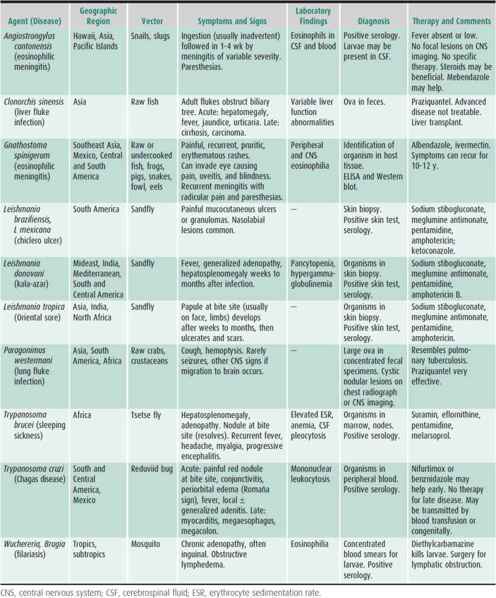
Selection of Patients for Evaluation
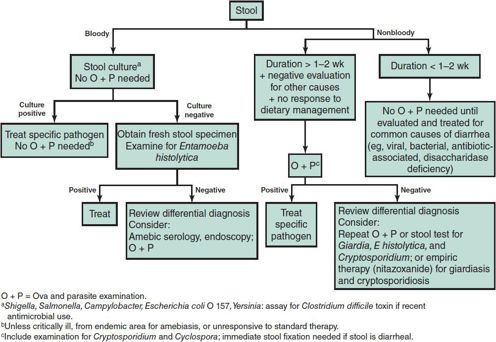
The incidence of parasitic infections varies greatly with geographic area. Children who have traveled or lived in areas where parasitic infections are endemic are at risk for infection with a variety of intestinal and tissue parasites. Children who have resided only in developed countries are usually free of tissue parasites (except Toxoplasma). Searching for intestinal parasites is expensive for the patient and time-consuming for the laboratory. More than 90% of ova and parasite examinations performed in most hospital laboratories in the United States are negative; many have been ordered inappropriately on patients without exposure to endemic areas. An approach to determining which children with diarrhea need such examinations is presented in Figure 43–1. It can be more cost-effective to empirically treat symptomatic US immigrants with albendazole for common intestinal parasites and to investigate only those whose symptoms persist.
Immunodeficient children are very susceptible to protozoal intestinal infections. Multiple opportunists are frequently identified, and the threshold for ordering tests should be low for these children.
Swanson SJ et al: Albendazole therapy and enteric parasites in United States-bound refugees. N Engl J Med 2012;366(16):1498–1507 [PMID: 22512482].
Specimen Processing
For tissue parasites, contact the laboratory for proper collection procedures. For intestinal parasites, diarrheal stools may contain trophozoites that die rapidly during transport. The specimen should be either examined immediately or placed in a stool fixative such as polyvinyl alcohol. Fixative vials for home collection of stool are commercially available. They may contain toxic compounds, so they should be stored safely. Fixed specimens are stable at room temperature. Formed stools usually contain cysts that are more stable. It is also best to fix these after collection, although they may be reliably examined after transport at room temperature. The US Centers for Disease Control and Prevention (CDC) have created a website (http://dpd.cdc.gov/dpdx) to assist in the laboratory diagnosis of common parasitic diseases, including specimen collection and processing.
Eosinophilia & Parasitic Infections
Although certain parasites commonly cause eosinophilia, in developed countries other causes are much more common. These include allergies, drugs, and other infections. Heavy intestinal nematode infection causing eosinophilia is easily detected on a single ova and parasite examination. Light nematode infections and common protozoal infections—giardiasis, cryptosporidiosis, and amebiasis—rarely cause eosinophilia. Eosinophilia is also unusual or minimal in more serious infections such as amebic liver abscess and malaria.
The most common parasitic infection in the United States that causes significant eosinophilia with negative stool examination is toxocariasis. In a young child with unexplained eosinophilia and a negative stool examination for parasites, a serologic test for Toxocara may be the next appropriate test. Trichinosis, which is a rare parasitic infection in the United States, causes marked eosinophilia. Strongyloidiasis is a cause of eosinophilia that may be difficult to diagnose with stool examinations. The differential diagnosis of eosinophilia is broad for patients who have been in developing countries (see Table 43–1).
PROTOZOAL INFECTIONS
SYSTEMIC INFECTIONS
1. Malaria
 General Considerations
General Considerations
Malaria causes approximately 1 million deaths each year, over 80% of which occur in children younger than 5 years of age in sub-Saharan Africa. Over the past 5 years, global efforts towards malaria prevention and treatment have led to declining mortality and morbidity. Approximately 1500 imported cases are diagnosed in the United States each year; local transmission may occasionally take place from imported cases. Human malaria is caused by five Plasmodium species—Plasmodium vivax (most common), Plasmodium falciparum (most virulent), Plasmodium ovale (similar to P vivax), Plasmodium malariae, and Plasmodium knowlesi (a primate parasite recently recognized as a cause of malaria in humans).
The female Anopheles mosquito transmits the parasites. An infected mosquito innoculates sporozoites into the bloodstream of a susceptible host, resulting in infection of hepatocytes. In the hepatic phase, the parasites mature into schizonts, which rupture and release merozoites into the circulation. The parasites infect and rupture red blood cells in the erthrocytic phase, as they mature from trophozoites to schizonts and release additional merozoites. In early stages of infection, asynchronous erythrocytic cycles of hemolysis commonly cause daily fevers. Eventually, synchronous erythrocytic cycles begin as parasites rupture the infected cells at more regular 48- or 72-hour intervals. Survival is associated with a progressive decrease in intensity of cycles. Relapses years later may occur from persistent hepatic infection, which occurs in P vivax and P ovale infections.
Susceptibility varies genetically; certain red cell phenotypes are partially resistant to P falciparum infection (hemoglobin S, hemoglobin F, thalassemia, and possibly glucose-6-phosphate dehydrogenase [G6PD] deficiency). The worldwide distribution of malaria species is determined to some extent by host genetic factors that have evolved in response to selective pressure from malaria. The absence of P vivax from Africa reflects the lack of specific Duffy blood group substances among most native Africans. Recurrent infections result in acquired species-specific immunity, which does not prevent infection, but decreases parasitemia and symptoms. Normal splenic function is an important factor because of the immunologic and filtration functions of the spleen. Asplenic persons develop rapidly progressive malaria with many circulating infected erythrocytes (including mature forms of P falciparum). Maternal immunity protects the neonate.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Clinical manifestations vary according to species, strain, and host immunity. Fever and vomiting are the most common presenting symptoms in children. Infants commonly present with recurrent bouts of fever, irritability, poor feeding, vomiting, jaundice, and splenomegaly. Rash is usually absent, which helps distinguish malaria from some viral infections in patients presenting with similar symptoms. In older children, the classic symptoms of fever with chills, rigors, headache, backache, myalgia, and fatigue are more easily elicited. Fever may be cyclic (every 48 hours for all but P malariae infection, in which it occurs every 72 hours) or irregular (most commonly observed with P falciparum). Between attacks, patients may look quite well. If the disease is untreated, relapses cease within a year in P falciparum and within several years in P vivax infections, but may recur decades later with P malariae infection. Infection during pregnancy often causes intrauterine growth restriction or premature delivery but rarely true fetal infection.
Physical examination in patients with uncomplicated cases may show only mild splenomegaly and anemia.
B. Laboratory Findings
The diagnosis of malaria relies on detection of one or more of the five human plasmodia in thick and thin blood smears. Three separate sets of thick and thin smears separated by 12–24 hours in a 72-hour period are recommended to rule out malaria infection. Thick smears are most sensitive for detection of small numbers of malaria parasites; thin smears allow identification of species and semiquantitative determination of percentage of parasitemia.
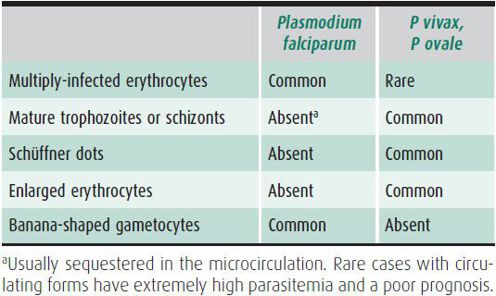
Most acute infections are caused by P vivax, P ovale, or P falciparum, although 5%–7% are due to multiple species. Identification of the Plasmodium species relies on morphologic criteria and requires an experienced observer (Table 43–3). Bench aids to assist in the identification of Plasmodium species can be found at http://www.dpd.cdc.gov/dpdx/HTML/Malaria.htm. A new US Food and Drug Administration (FDA)-approved antigen detection test is available and approved for rapid diagnostic testing of malaria. This test should be used in conjunction with microscopic examination to confirm diagnosis, look for mixed infection, and quantitate degree of parasitemia. The rapid antigen test has poor sensitivity for low levels of parasitemia. Up-to-date information on rapid diagnostic testing for malaria can be found at www.cdc.gov/malaria/diagnosis_treatment/index.html. Alternative techniques of similar or higher diagnostic accuracy for P falciparum include DNA hybridization and polymerase chain reaction (PCR), which are only available in research and reference laboratories as well as at CDC and some health departments.
Table 43–3. Differentiation of malaria parasites on blood smears.
The degree of parasitemia determined from thin smears is particularly useful in the management of infections caused by P falciparum and P knowlesi, in which high parasitemia (> 5% infected erythrocytes) is associated with high morbidity and mortality and requires hospitalization. Treatment response of P falciparum and chloroquine-resistant P vivax infections is best monitored by daily parasitemia assays. Constant or increased number of infected erythrocytes after 48 hours of treatment or after the second hemolytic crisis suggests an inadequate therapeutic response.
Hemolytic anemia and thrombocytopenia are common; the incidence of leukocytosis is variable.
 Differential Diagnosis
Differential Diagnosis
Relapsing fever may be associated with borreliosis, brucellosis, sequential common infections, Hodgkin disease, juvenile rheumatoid arthritis, or rat-bite fever. Other common causes of high fever and headache include influenza, Mycoplasma pneumoniae or enteroviral infection, sinusitis, meningitis, enteric fever, tuberculosis, occult pneumonia, or bacteremia. In patients returning from tropical areas with fever, headache, and jaundice, leptospirosis and yellow fever should be included in the differential diagnosis. Clinical features may not reliably distinguish severe malaria from other severe infections in children, so a high index of suspicion in patients with exposure in endemic areas is necessary. Malaria may also coexist with other diseases.
 Complications & Sequelae
Complications & Sequelae
Severe complications, which are limited to P falciparum and P knowlesi infection, result from hemolysis, microvascular obstruction, and tissue ischemia. The most common complications of malaria in children are cerebral malaria, respiratory distress, severe anemia, and/or hypoglycemia. Cerebral malaria is the most serious and life-threatening complication of malaria in children and may progress to seizures, coma, and death. Approximately 20% of children with cerebral malaria die and 10% have long-term neurologic sequelae. Signs of severe malaria in children include altered mental status, seizures, respiratory distress, hypoglycemia, acidosis, and parasitemia greater than 5%.
 Prevention
Prevention
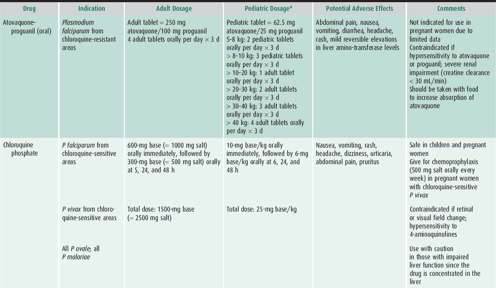
Malaria chemoprophylaxis should be instituted 2 weeks (weekly regimens) to 2 days (daily regimens) before traveling to an area of endemic infection to permit alternatives if the drug is not tolerated. Because the antimalarial drugs recommended for prophylaxis do not kill sporozoites, therapy should be continued for 1 week (atovaquone-proguanil) or 4 weeks (all other regimens) after returning from an endemic area to cover infection acquired at departure.
No drug regimen guarantees protection against malaria. If fever develops within 1 year (particularly within 2 months) after travel to an endemic area, the possibility of malaria should be considered. Insect repellents, insecticide-impregnated bed nets, and proper clothing are important adjuncts for malaria prophylaxis.
For a full discussion regarding currently recommended medications for malaria prophylaxis, please see the corresponding section in Chapter 45.
 Treatment
Treatment
Treatment for malaria includes a variety of supportive strategies in addition to the antimalarial drugs. It is advisable to hospitalize nonimmune patients infected with P falciparum and P. knowlesi until a decrease in parasitemia is demonstrated, indicating that treatment is effective and severe complications are unlikely to occur. Patients with signs of severe malaria (parasitemia > 5%, cerebral malaria, acidosis, hypoglycemia, shock) require intensive care and parenteral treatment. Malaria due to P knowlesi should be treated like P falciparum malaria as it might progress rapidly into severe disease.
Partially immune patients with uncomplicated P falciparum and P knowlesi infection and nonimmune persons infected with P vivax, P ovale, or P malariae can receive treatment as outpatients if follow-up is reliable. For children, hydration and treatment of hypoglycemia are of utmost importance. Anemia, seizures, pulmonary edema, and renal failure require conventional management. Corticosteroids are contraindicated for cerebral malaria because of increased mortality. In severe malaria, exchange transfusion can be lifesaving, particularly in nonimmune persons with parasitemia greater than 10%.
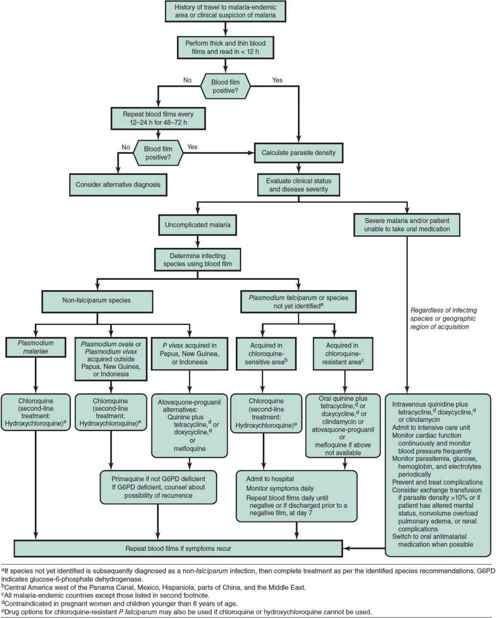
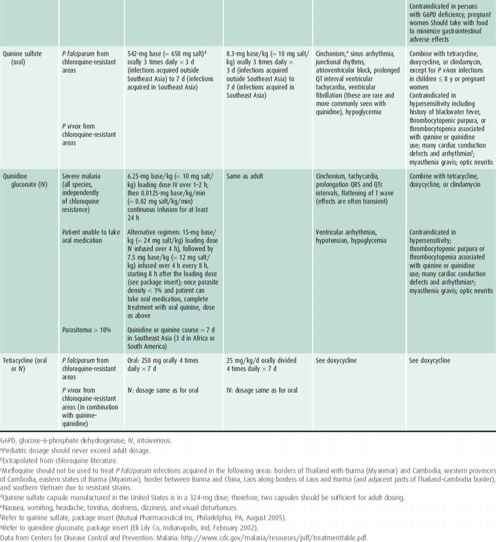
Choice of antimalarial treatment depends on the immune status of the person, plasmodium species, degree of parasitemia, and resistance patterns in the geographical region of acquisition. An algorithm for the treatment of malaria is shown in Figure 43–2 and a description of the recommended antimalarial drugs available in the United States is provided in Table 43–4. Updated treatment guidelines are available at the Centers for Disease Control and Prevention (http://www.cdc.gov/malaria/diagnosis_treatment/treatment.html). Artemisinin derivatives clear parasites very rapidly and are widely used as key components, often as first-line therapy, in malaria treatment worldwide. These are available in the United States for the treatment of severe malaria only by contacting the CDC (http://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html) or the Malaria Hotline at 770-488-7788.
 Figure 43–2. Malaria treatment algorithm. (Adapted from Centers for Disease Control and Prevention. Malaria. http://www.cdc.gov/malaria/resources/pdf/algorithm.pdf.
Figure 43–2. Malaria treatment algorithm. (Adapted from Centers for Disease Control and Prevention. Malaria. http://www.cdc.gov/malaria/resources/pdf/algorithm.pdf.
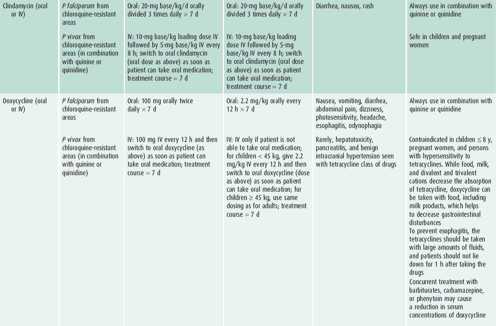
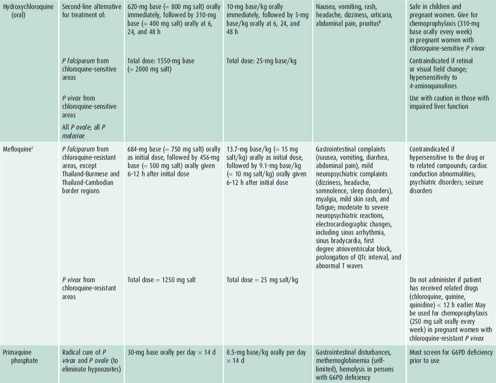
Table 43–4. Antimalarial drugs available in the United States recommended for use in the treatment of malaria.
Crawley J et al: Malaria in children. Lancet 2010;375:1468–1481 [PMID: 20417858].
Griffith KS et al: Treatment of malaria in the United States. JAMA 2007;297:2264 [PMID: 17519416].
http://www.dpd.cdc.gov/dpdx/HTML/Malaria.htm/.
http://www.who.int/malaria/diagnosis_treatment/.
Rosenthal PJ: Artesunate for the treatment of severe falciparum malaria. N Engl J Med 2008;358:1829–1836 [PMID: 18434652].
Taylor SM et al: Does this patient have malaria? JAMA 2010;304:2048–2056 [PMID: 21057136].
2. Babesiosis
Babesia microti is a malaria-like protozoan that infects humans bitten by infected Ixodes scapularis (deer tick), one of its intermediate hosts and vectors. After inoculation, the protozoan penetrates erythrocytes and starts an asynchronous cycle that causes hemolysis. In the United States, the majority of cases occur in the Northeast and upper Midwest from May to October. Babesia infection is also a transfusion-transmissible disease.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The incubation period is 1–4 weeks after tick bite, or 1–9 weeks after blood transfusion. Many times the tick bite is unnoticed. Approximately half of infected children are asymptomatic. Symptoms are nonspecific and most commonly include sustained or cyclic fever up to 40.9°C, shaking chills, and sweats. Other associated nonspecific symptoms include malaise, fatigue, anorexia, arthralgias, myalgias, and headache. Physical examination findings are usually minimal, but may include hepatosplenomegaly, jaundice, or dark urine. The disease is usually self-limited, causing symptoms for 1–2 weeks with fatigue that may persist for months. Severe cases have been described in asplenic patients and immunocompromised hosts. Because Babesia, Borrelia burgdorferi, and Anaplasma phagocytophilum share a common vector, physicians should consider the possibility of coinfection in patients diagnosed with any of these pathogens.
B. Laboratory Findings
The diagnosis is made by identifying babesial parasites in blood by microscopic evaluation of thin or thick blood smears or by PCR amplification of babesial DNA. Babesia parasites are intraerythrocytic organisms that resemble P falciparum ring forms. The tetrad form (Maltese cross), if visualized, is pathognomonic. Specific serologic tests are also available through the CDC. Hemolytic anemia, as well as thrombocytopenia, is common.
 Treatment
Treatment
Azithromycin (10 mg/kg up to 500 mg on the first day, followed by 5 mg/kg up to 250 mg/day) in combination with atovaquone (20 mg/kg, up to 750 mg, twice a day) for 7–10 days is the treatment of choice for mild to moderate disease. It has been found to be efficacious and causes fewer adverse side effects than other regimens. For severely ill patients, clindamycin (10 mg/kg, up to 600 mg, every 8 hours) in combination with quinine (8 mg/kg, up to 650 mg, every 8 hours) is standard of care. Longer courses of treatment may be needed in immunocompromised patients. Partial or complete RBC exchange transfusion is indicated for persons with severe babesiosis, as indicated by high-grade parasitemia (≥ 10%); significant hemolysis; or renal, hepatic, or pulmonary compromise.
Gubernot DM et al: Babesia infection through blood transfusion: reports received by the US Food and Drug Administration, 1997-2007. Clin Infect Dis 2009;48:25–30 [PMID: 19035776].
http://www.cdc.gov/parasites/babesiosis/.
http://www.dpd.cdc.gov/dpdx/HTML/Babesiosis.htm.
Vannier E, Krause PJ: Human babesiosis. N Engl J Med 2012;366:2397–2407 [PMID: 22716978].
3. Toxoplasmosis
 General Considerations
General Considerations
Toxoplasma gondii is a worldwide parasite of animals and birds. Felines, the definitive hosts, excrete oocysts in their feces. Ingested mature oocysts or tissue cysts lead to tachyzoite invasion of intestinal cells. Intracellular replication of the tachyzoites causes cell lysis and spread of the infection to adjacent cells or to other tissues via the bloodstream. In chronic infection, T gondii appears in bradyzoite-containing tissue cysts that do not trigger an inflammatory reaction. In immunocompromised hosts, tachyzoites are released from cysts and begin a new cycle of infection.
The two major routes of Toxoplasma transmission to humans are oral and congenital. Oral infection occurs after ingestion of cysts from food, water, or soil contaminated with cat feces or from ingestion of undercooked meat or other food products that contain cysts. Oocysts survive for up to 18 months in moist soil. Oocyst survival is limited in dry, very cold, or very hot conditions, and at high altitude, which probably accounts for the lower incidence of toxoplasmosis in these climatic regions. In the United States, less than 1% of cattle and 25% of sheep and pigs are infected with toxoplasmosis. In humans, depending on geographic area, seropositivity increases with age from 0 to 10% in children younger than age 10 years to 3%–70% in adults.
Congenital transmission occurs during acute infection of pregnant women. Rarely, fetal infection has been documented in immunocompromised mothers who have chronic toxoplasmosis. Treatment during pregnancy decreases transmission by 60%.
 Clinical Findings
Clinical Findings
Clinical toxoplasmosis can be divided into four groups: (1) congenital infection, (2) acquired in the immunocompetent host, (3) acquired or reactivated in the immunocompromised host, and (4) ocular disease.
A. Congenital Toxoplasmosis
Congenital toxoplasmosis is the result of acute infection during pregnancy and occurs in 1 in 3000 to 1 in 10,000 live births in the United States. The rate of transmission and disease severity in the baby vary according to when in pregnancy the infection is acquired. First-trimester infections lead to congenital infections about 10%–20% of the time, but the clinical disease is severe with microcephaly or hydrocephaly, severe chorioretinitis, hearing loss, convulsions, abnormal cerebrospinal fluid (CSF) (xanthochromia and mononuclear pleocytosis), cerebral calcifications, and mental retardation. Other findings include strabismus, eye palsy, maculopapular rash, pneumonitis, myocarditis, hepatosplenomegaly, jaundice, thrombocytopenia, lymphocytosis and monocytosis, and an erythroblastosis-like syndrome. Infection of a mother in the third trimester results in 70%–90% rate of congenital infection, but the majority of these children are asymptomatic at birth. Many of these children, however, will go on to develop ocular disease later and some will have subtle neurologic deficits.
B. Acquired Toxoplasma Infection in the Immunocompetent Host
Typically, acquired infection in the immunocompetent host is asymptomatic. About 10%–20% of patients develop lymphadenopathy and/or a flu-like illness. The nodes are discrete, variably tender, and do not suppurate. Cervical lymph nodes are most frequently involved, but any nodes may be enlarged. Less common findings include fever, malaise, myalgias, fatigue, hepatosplenomegaly, low lymphocyte counts (usually < 10%), and liver enzyme elevations. Unilateral chorioretinitis may occur. The disease is self-limited, although lymph node enlargement may persist or may wax and wane for a few months to 1 or more years.
Toxoplasmic lymphadenitis must be distinguished from other causes of infectious mononucleosis-like syndromes (< 1% are caused by Toxoplasma). Recovery typically occurs without any specific antiparasitic treatment.
C. Acute Toxoplasmosis in the Immunodeficient Host
Patients infected with human immunodeficiency virus (HIV), and those with lymphoma, leukemia, or transplantation, are at high risk for developing severe disease (most commonly central nervous system [CNS] disease, but also chorioretinitis, myocarditis, or pneumonitis) following acute infection or reactivation. Toxoplasmic encephalitis is one of the most common causes of mass lesions in the brains of persons with HIV/AIDS.
D. Ocular Toxoplasmosis
Ocular toxoplasmosis is an important cause of chorioretinitis in the United States. It can result from reactivation of congenital infection or acquired infection. Congenitally infected individuals are usually asymptomatic until the second or third decade of life when symptomatic eye disease occurs due to the rupture of tissue cysts and the release of bradyzoites and tachyzoites into the retina. Typically, ocular toxoplasmsis presents as a focal necrotizing retinochoroiditis often associated with a preexistent chorioretinal scar, and variable involvement of the vitreous, retinal blood vessels, optic nerve, and anterior segment of the eye. The appearance of the ocular lesion is not specific and mimics other granulomatous ocular diseases.
E. Diagnostic Findings
Serologic tests are the primary means of diagnosis, but results must be interpreted carefully. Reference laboratories with special expertise in toxoplasma serologic assays and their interpretation are essential for establishing a diagnosis and are preferred. Active infection can also be diagnosed by PCR of blood or body fluids; by visualization of tachyzoites in histologic sections or cytology preparations, cysts in placenta or fetal tissues; or by characteristic lymph node histology. IgG antibodies become detectable 1–2 weeks after infection, peak at 1–2 months, and thereafter persist for life. IgM antibodies, measured by ELISA (enzyme-linked immunosorbent assay) or particle agglutination, appear earlier and decline faster than IgG antibodies, but can last for 12–18 months after acute infection. Absence of both serum IgG and IgM virtually rules out the diagnosis of toxoplasmosis. Acute toxoplasmosis in an immunocompetent host is best documented by analyzing IgG and IgM in paired blood samples drawn 3 weeks apart. Because high antibody titers (IgM or IgG) can persist for several months after acute infection, a single high-titer determination is nondiagnostic; seroconversion or a fourfold increase in titer confirms the diagnosis. In the immunocompromised host, serologic tests are not sensitive, and active infection is documented by PCR or finding tachyzoites by histologic examination.
The diagnosis of toxoplasmosis in the older child with visual complaints is usually made by finding T gondii IgG or IgM antibodies in the serum combined with the presence of a typical eye lesion. The diagnosis can be confirmed by detecting T gondii DNA by PCR in the aqueous humor.
Diagnosis of congenital infection, which can be difficult, is made by a combination of serologic testing, parasite isolation, and clinical findings. Prenatally, diagnosis can be made by PCR of amniotic fluid. Postnatally, evaluation of the baby should include Toxoplasma-specific IgG, IgM, IgA, and IgE of the newborn and mother. IgM antibodies indicate an immune response by the infant. Persistence of the other antibodies also indicates infection of the infant. Blood, CSF, and amniotic fluid specimens should be assayed by PCR for the presence of T gondii in a reference laboratory. In addition, the child should have thorough ophthalmologic, auditory, and neurologic evaluation; a lumbar puncture; and computed tomographic (CT) scan of the head (to detect CNS calcifications). A congenital infection is confirmed serologically by detecting persistent or increasing IgG antibody levels compared to the mother, persistently positive IgG antibodies beyond the first year of life, and/or a positive T gondii–specific IgM or IgA antibody test.
 Differential Diagnosis
Differential Diagnosis
Congenital toxoplasmosis must be differentiated from infection with cytomegalovirus, rubella, herpes simplex, syphilis, listeriosis, erythroblastosis, and the encephalopathies that accompany degenerative diseases. Acquired infection can mimic viral, bacterial, or lymphoproliferative disorders. Ocular toxoplasmosis can mimic other infectious, noninfectious, and neoplastic ocular conditions.
 Prevention
Prevention
Primary prevention of toxoplasmosis in pregnant women (and immunocompromised patients) is an essential public health goal and guidelines have been published by the CDC. These include (1) cook meat until well-done 150°F–170°F (no longer pink in center); (2) peel and/or wash fruits and vegetables thoroughly before eating; (3) wash hands, cutting boards, kitchen surfaces, and utensils after contact with raw meat; (4) avoid drinking untreated water from unsanitary sources; (5) wear gloves when gardening or coming in contact with soil or sand, as cat feces may contaminate them; and (6) if possible, pregnant women should avoid changing cat litter. If they cannot avoid changing cat litter, they should change it daily as oocysts require 48–72 hours to sporulate and become infectious. Serologic screening of pregnant women remains a controversial tool in the prevention of congenital toxoplasmosis.
 Treatment
Treatment
Treatment of acute toxoplasmosis in the immunocompetent host does not require specific therapy, unless the infection occurs during pregnancy. Toxoplasmic chorioretinitis presenting beyond infancy is treated the same way regardless of whether it is due to reactivation or primary infection. Treatment consists of oral pyrimethamine (2 mg/kg maximum 200 mg loading dose followed by 1 mg/kg daily, maximum 75 mg) plus sulfadiazine (100 mg/kg daily, maximum 1500 mg), given with leucovorin (10–20 mg three times a week). In addition, corticosteroids (prednisone 1 mg/kg daily) are given when lesions threaten vision. Treatment is given for 1–2 weeks after the resolution of symptoms. The duration of additional therapy should be guided by frequent ophthalmologic examinations. Pyrimethamine can cause gastrointestinal upset, leukopenia, thrombocytopenia, and rarely, agranulocytosis; weekly complete blood counts should be checked while on therapy.
A year of treatment is recommended for all congenitally infected infants. Children treated with pyrimethamine (loading dose 2 mg/kg daily for 2 days followed by 1 mg/kg daily for 6 months followed by 1 mg/kg every Monday, Wednesday, and Friday for 6 months) plus sulfadiazine (100 mg/kg divided twice daily for 12 months) plus leucovorin (10 mg 3 times a week) have better neurodevelopmental and visual outcomes than historical controls. While on therapy, infants should be monitored for bone marrow toxicity.
In primary maternal infection during the first 18 weeks of pregnancy, spiramycin is recommended to attempt to prevent fetal infection. Spiramycin does not cross the placenta, so does not treat fetal infection. If fetal infection has been documented or if primary maternal infection occurs after the first 18 weeks of pregnancy, pyrimethamine, sulfadiazine, and leucovorin are recommended. Pyrimethamine is teratogenic and should not be used before 18 weeks of gestation.
http://www.cdc.gov/parasites/toxoplasmosis/.
McAuley JB: Toxoplasmosis in children. Pediatr Infect Dis J 2008;27:161–162 [PMID: 18227714].
Montoya JG, Remington JS: Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008;47(4):554–566 [PMID: 18624630].
Olariu TR et al: Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 2011;30(12):1056–1061 [PMID: 21956696].
Vasconcelos-Santos DV et al: Review for disease of the year: differential diagnosis of ocular toxoplasmosis. Ocul Immunol Inflamm 2011;19:171–179 [PMID: 21595533].
GASTROINTESTINAL INFECTIONS
1. Amebiasis
 General Considerations
General Considerations
Amebiasis is defined as an infection with Entamoeba histolytica regardless of symptoms. This parasite is found worldwide, but has a particularly high prevalence in areas with poor sanitation and socioeconomic conditions. In the United States, infections are seen in travelers to and emigrants from endemic areas. The majority of infections are asymptomatic (> 90%), but tissue invasion can result in amebic colitis, hepatic abscess, and hematogenous spread to other organs. Transmission is usually fecal-oral. Infections with two other Entamoeba species, E dispar and E moshkovskii, are morphologically indistinguishable from E histolytica. Infections with these species are approximately 10 times more common than infection with E histolytica, but are not thought to cause human disease.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Patients with intestinal amebiasis can have asymptomatic cyst passage, or be symptomatic with acute amebic proctocolitis, chronic nondysenteric colitis, or ameboma. Because all E dispar and E moshkovskii infections and up to 90% of E histolytica infections are asymptomatic, carriage is the most common manifestation of amebiasis. Patients with acute amebic colitis typically have a 1- to 2-week history of loose stools containing blood and mucus, abdominal pain, and tenesmus. A minority of patients are febrile or dehydrated. Abdominal examination may reveal pain over the lower abdomen.
Fulminant colitis is an unusual complication of amebic dysentery that is associated with a grave prognosis (> 50% mortality), and is characterized by severe bloody diarrhea, fever, and diffuse abdominal pain. Children younger than age 2 years are at increased risk for this condition. Chronic amebic colitis causes recurrent episodes of bloody diarrhea over a period of years and is clinically indistinguishable from idiopathic inflammatory bowel disease. An ameboma is a localized amebic infection, usually in the cecum or ascending colon, which presents as a painful abdominal mass.
The most common complications of intestinal amebiasis are intestinal perforation, toxic megacolon, and peritonitis. Perianal ulcers, a less common complication, are painful, punched-out lesions that usually respond to medical therapy. Infrequently, colonic strictures may develop following colitis.
Extraintestinal amebiasis can result in liver, lung, and cerebral abscesses, and rarely genitourinary disease. Patients with amebic liver abscess, the most common form of extraintestinal amebiasis, typically present with acute fever and right upper quadrant tenderness. The pain may be dull, pleuritic, or referred to the right shoulder. Physical examination reveals liver enlargement in less than 50% of affected patients. Some patients have a subacute presentation lasting 2 weeks to 6 months. In these patients, hepatomegaly, anemia, and weight loss are common findings, and fever is less common. Jaundice and diarrhea are rarely associated with an amebic liver abscess. In children with fever of unknown origin who live in, or travel to, endemic areas, amebic liver abscess should be considered in the differential diagnosis.
The most common complication of amebic liver abscess is pleuropulmonary amebiasis due to rupture of a right liver lobe abscess. Lung abscesses may occur from hematogenous spread. Cough, dyspnea, and pleuritic pain can be caused by the serous pleural effusions and atelectasis that frequently accompany amebic liver abscesses. Rupture of hepatic abscesses can lead to peritonitis and more rarely to pericarditis. Amebic brain abscess is an infrequent manifestation.
B. Diagnostic Findings
The differential diagnosis of acute amebic colitis includes bacterial (eg, Salmonella spp, Shigella spp, E coli spp, Campylobacter spp), parastitic (eg, Schistosoma mansoni, Balantidium coli), and noninfectious (eg, inflammatory bowel disease, diverticulitis, ischemic colitis) causes of dysentery. Chronic amebic colitis has to be distinguished from inflammatory bowel disease and Cyclospora. Occult blood is present in virtually all cases of amebic colitis and can be used as an inexpensive screening test. Fecal leukocytes are uncommon.
Intestinal amebiasis can be presumptively diagnosed by detecting the parasite on stool examination or mucosal biopsy. However, E histolytica is morphologically identical to nonpathogenic E dispar and E moshkovskii and cannot be easily differentiated by microscopy. The presence of trophozoites with ingested red blood cells in feces is more suggestive of pathogenic E histolytica infection. Ideally, a wet mount preparation of the stool should be examined within 20 minutes after collection to detect motile trophozoites. Otherwise, specimens should be fixed with polyvinyl alcohol or refrigerated to avoid disintegration of the trophozoites. The antigen detection test for stool is very sensitive and more specific, because it detects E histolytica–specific antigens that do not cross-react with E dispar. Colonoscopy and biopsy are most helpful in diagnosing amebic colitis when stool samples lack ova or parasites. Barium studies are contraindicated for patients with suspected acute amebic colitis because of the risk of perforation.
The presence of antibodies against E histolytica can differentiate E histolytica from E dispar infections. Specific antibody is induced by both intestinal and extraintestinal invasive amebiasis. ELISA assays are positive in approximately 95% of patients with extraintestinal amebiasis, 70% with intestinal E histolytica disease, and 10% of asymptomatic patients shedding E histolytica cysts. However, these antibodies persist for years, and a positive result does not distinguish between acute and past infection, but are useful in a traveler from a nonendemic area who is returning from a trip to an endemic area. Ultrasonographic examination and CT are sensitive techniques to detect hepatic abscesses and can be used to guide fine-needle aspiration to obtain specimens for definitive diagnosis. Because an amebic abscess may take up to 2 years to completely resolve on CT scans, imaging techniques are not recommended for therapeutic evaluation. PCR-based testing has the highest sensitivity and specificity for the diagnosis of E histolytica, but is only available in certain research and reference laboratories.
 Prevention & Treatment
Prevention & Treatment
Travelers to endemic areas need to follow the precautions for preventing enteric infections—drink bottled or boiled water and eat cooked or peeled vegetables and fruits.
Treatment of amebic infection is complex because different agents are required for eradicating the parasite from the bowel or tissue (Table 43–5). Whether treatment of asymptomatic cyst passers is indicated is a controversial issue. The prevalent opinion is that asymptomatic infection with E histolytica, as evidenced by amebic cysts in the stool and a positive serologic test, should be treated in nonendemic areas. If serologic tests are negative, the cysts are more likely to represent infection with the nonpathogenic E dispar, which does not require treatment.
Table 43–5. Treatment of amebiasis.
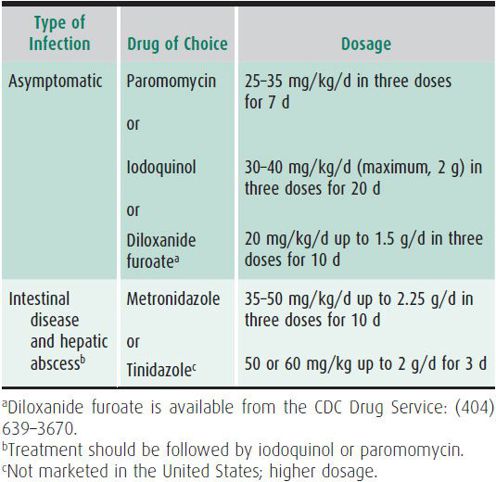
Asymptomatic E histolytica cyst excreters may be treated with paromomycin, a nonabsorbable intraluminal amebicide. Diloxanide and iodoquinol are alternative intraluminal agents. Metronidazole is not effective against cysts.
Patients with symptomatic intestinal amebiasis or extraintestinal disease require treatment with an absorbable agent, such as metronidazole or tinidazole, followed by an intraluminal agent. Metronidazole has a disulfiram-like effect and should be avoided in patients receiving ethanol-containing medications. Tinidazole, a more potent nitroimidazole against amebic infection, can be used for shorter treatment courses and is well tolerated in children. Treatment of invasive amebiasis should always be followed with an intraluminal cysticidal agent, even if the stool examination is negative. Metronidazole and paromomycin should not be given concurrently, because the diarrhea that is a common side effect of paromomycin may make it difficult to assess response to therapy. In most patients with amebic liver abscess, aspiration is unnecessary and does not speed recovery. Patients with large, thin-walled hepatic abscesses may need therapeutic aspiration to avoid abscess rupture. Drainage may also be considered when response to medical therapy is inadequate.
Fotedar R et al: Laboratory diagnostic techniques for Entamoeba species. Clin Micro Rev 2007;20:511–532 [PMID: 17630338].
Haque R et al: Amebiasis. N Engl J Med 2003;348:1565–1573 [PMID: 12700377].
http://www.dpd.cdc.gov/dpdx/html/amebiasis.htm.
Stanley SL et al: Amoebiasis. Lancet 2003;361:1025–1034 [PMID: 12660071].
2. Giardiasis
 General Considerations
General Considerations
Giardiasis, caused by Giardia intestinalis (formerly Giardia lamblia), is the most common intestinal protozoal infection in children in the United States and in most of the world. The infection is classically associated with drinking contaminated water, either in rural areas or in areas with faulty purification systems. Even ostensibly clean urban water supplies can be contaminated intermittently, and infection has been acquired in swimming pools. Fecal-oral contamination allows person-to-person spread. Day care centers are a major source of infection, with an incidence of up to 50% reported in some centers. No symptoms occur in 25% of infected persons, facilitating spread to household contacts. Food-borne outbreaks also occur. Although infection is rare in neonates, giardiasis may occur at any age.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Giardia infection is followed by either asymptomatic cyst passage, acute self-limited diarrhea, or a chronic syndrome of diarrhea, malabsorption, and weight loss. Acute diarrhea occurs 1–2 weeks after infection and is characterized by abrupt onset of diarrhea with greasy, malodorous stools; malaise; flatulence; bloating; and nausea. Fever and vomiting are unusual. The disease has a protracted course (> 1 week) and frequently leads to weight loss. Giardiasis can be a self-limited infection in some patients, and in others cause chronic symptoms. Patients who develop chronic diarrhea complain of profound malaise, lassitude, headache, and diffuse abdominal pain in association with bouts of diarrhea—most typically foul-smelling, greasy stools—intercalated with periods of constipation or normal bowel habits. This syndrome can persist for months until specific therapy is administered or until it subsides spontaneously. Chronic diarrhea frequently leads to malabsorption, steatorrhea, vitamins A and B12 deficiencies, and disaccharidase depletion. Lactose intolerance, which develops in 20%–40% of patients can persist for several weeks after treatment, and needs to be differentiated from relapsing giardiasis or reinfection.
B. Laboratory Findings
Giardia antigen detection by means of ELISAs, nonenzymatic immunoassays, and direct fluorescence antibody tests are the standard diagnostic tests in the United States. They have a more rapid return of results, and are more sensitive and specific than stool ova and parasite examination. In resource-poor areas without access to antigen tests, the diagnosis of giardiasis can be made by finding the parasite in stool. For ova and parasite examination, a fresh stool provides the best results. Liquid stools have the highest yield of trophozoites, which are more readily found on wet mounts. With semiformed stools, the examiner should look for cysts in fresh or fixed specimens, preferably using a concentration technique. When these techniques are applied carefully, one examination has a sensitivity of 50%–70%; three examinations increase the sensitivity to 90%. With a careful stool ova and parasite examination or with the use of a new antigen test, direct sampling of duodenal aspirates or biopsy, which was utilized in the past, is now restricted to particularly difficult cases.
 Prevention
Prevention
The prevention of giardiasis requires proper treatment of water supplies and interruption of person-to-person transmission. Where water might be contaminated, travelers, campers, and hikers should use methods to make water safe for drinking. Boiling is the most reliable method; the necessary time of boiling (1 minute at sea level) will depend on the altitude. Chemical disinfection with iodine or chlorine and filtration are alternative methods of water treatment.
Interrupting fecal-oral transmission requires strict hand washing. However, outbreaks of diarrhea in day care centers might be particularly difficult to eradicate. Reinforcing hand washing and treating the disease in both symptomatic and asymptomatic carriers may be necessary.
 Treatment
Treatment
Metronidazole, tinidazole, and nitazoxanide are the drugs of choice for treatment of giardiasis. When given at 5 mg/kg (up to 250 mg) three times a day for 5–7 days, metronidazole has 80%–95% efficacy. The drug is well tolerated in children. Tinidazole has an efficacy approaching 90% when given as a single dose of 50 mg/kg (up to 2 g). Nitazoxanide is available in liquid formulation and requires only 3 days of treatment. Recommended doses are 100 mg (5 mL) every 12 hours for children 12–47 months of age, 200 mg (10 mL) every 12 hours for 4- to 11-year-olds, and 500 mg every 12 hours for children 12 years or older. Furazolidone is sometimes used in children because it is available in suspension. Administered at 1.5 mg/kg (up to 100 mg) four times daily for 7–10 days, it has only 80% efficacy. Furazolidone may cause gastrointestinal side effects, turn urine red, and cause mild hemolysis in patients with G6PD deficiency. For patients who do not respond to therapy, or are re-infected, a second course with the same drug or switching to another drug is equally effective. In cases of repeated treatment failure, albendazole (400 mg/d for 5–10 days), although not specifically recommended in the United States for the treatment of giardiasis, is an effective option.
Escobedo AA et al: Giardiasis: the ever-present threat of a neglected disease. Infect Disord Drug Targets 2010;10(5):329–348 [PMID: 20701575].
http://www.cdc.gov/parasites/giardia/.
Huang DB et al: An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am 2006;35:291 [PMID: 16880067].
3. Cryptosporidiosis
Cryptosporidia are intracellular protozoa that gained importance because they cause severe and devastating diarrhea in patients with acquired immunodeficiency syndrome (AIDS) and in other immunodeficient persons. This ubiquitous parasite infects and reproduces in the epithelial cell lining of the digestive and respiratory tracts of humans and most other vertebrate animals. Humans acquire the infection from contaminated drinking water, recreation water sources (including swimming pools, fountains, and lake water), or from close contact with infected humans or animals. Cryptosporidia are the leading cause of recreational water-associated outbreaks in the United States. Petting zoos and day care centers have been other sources of Cryptosporidia outbreaks. Most human infections are caused by C parvum or C hominis, although other species have been reported to cause human disease.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Immunocompetent persons infected with Cryptosporidium spp usually develop self-limited diarrhea (2–26 days) with or without abdominal cramps. Diarrhea can be mild and intermittent or continuous, watery, and voluminous. Low-grade fever, nausea, vomiting, loss of appetite, and malaise may accompany the diarrhea. Children younger than age 2 years are more susceptible to infection than older children. Immunocompromised patients (either cellular or humoral deficiency) tend to develop a severe, prolonged, chronic diarrhea which can often result in severe malnutrition, and subsides only after the immunodeficiency is corrected. Other clinical manifestations associated with cryptosporidiosis in immunocompromised hosts include cholecystitis, pancreatitis, hepatitis, biliary tree involvement, and respiratory symptoms.
B. Laboratory Findings
Visualization of Cryptosporodia oocysts in the stool is diagnostic. Direct immunofluorescent antibody (DFA) of stool is the test of choice for visualizing oocysts. Oocysts can also be visualized with a modified Kinyoun acid-fast stain on concentrated stool. ELISA and point-of-care rapid tests are commercially available, but should be confirmed by microscopy.
 Prevention & Treatment
Prevention & Treatment
Prevention of Cryptosporidium infection is limited by oocyst resistance to some of the standard water purification procedures and to common disinfectants. Enteric precautions are recommended for infected persons. Boiled or bottled drinking water may be considered for those at high risk for developing chronic infection (eg, inadequately treated patients with AIDS).
Immunocompetent patients and those with temporary immunodeficiencies respond to treatment with nitazoxanide, antidiarrheal agents, and hydration. Immunocompromised patients usually require more intense supportive care with parenteral nutrition in addition to hydration and nonspecific antidiarrheal agents. Octreotide acetate, a synthetic analogue of somatostatin that inhibits secretory diarrhea, has been associated with symptomatic improvement but not with parasitologic cure. Nitazoxanide for 3 days is the treatment of choice; recommended doses are 100 mg (5 mL) every 12 hours for children 12–47 months of age, 200 mg (10 mL) every 12 hours for 4- to 11-year-olds, and 500 mg every 12 hours for children 12 years or older. For patients with advanced AIDS, antiparasitic therapy alone has not proven efficacious. Institution of effective anti-retroviral therapy results in elimination of symptomatic cryptosporidiosis.
Cabada MM et al: Treatement of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis 2010;23:494–499 [PMID: 20689422].
Davies AP et al: Cryptosporidiosis. BMJ 2009;399:963–967 [PMID: 19841008].
http://www.cdc.gov/parasites/crypto/.
Huang DB et al: An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am 2006;35:291 [PMID: 16880067].
4. Cyclosporiasis
Cyclospora spp are ubiquitous coccidian parasites that infect both humans and a variety of animals worldwide. Cyclospora cayetanensis is the only species known to infect humans. Cyclosporiasis is seen in three main epidemiologic settings: sporadic cases in endemic areas (particularly Haiti, Guatemala, Peru, and Nepal), travelers to endemic areas, and in food- or water-borne outbreaks in nonendemic areas, particularly in relation to importation of fresh produce. The incubation period is approximately 7 days (range 2–14 days). Infection may be asymptomatic, cause mild to moderate self-limiting diarrhea, or cause protracted or severe diarrhea. In the immunocompetent host, diarrhea usually lasts 10–25 days but may be followed by a relapsing pattern that can last several months. Diarrhea (5–15 movements per day) is usually watery, sometimes explosive, and often accompanied by nausea, vomiting, abdominal cramping, and bloating. Profound fatigue, anorexia, and myalgias have been reported. The infection can be unusually severe in immunocompromised patients, especially those with HIV/AIDS. Although the illness is self-limited, it may last for several weeks. Diagnosis is based on finding oocysts 8–10 mm in diameter on examination of stool specimens stained with acid-fast stain. PCR of stool is available at the CDC and some reference laboratories. The treatment of choice is trimethoprim-sulfamethoxazole for 7 days.
Ortega YR et al: Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Micro Rev 2011;23:218–234 [PMID: 20065331].
5. Free-Living Amoebas
 General Considerations
General Considerations
Infections with free-living amoebas are uncommon. Naegleria species, Acanthamoeba species, and Balamuthia amoebas have been associated with human disease, primarily infections of the central nervous system.
Acute meningoencephalitis, caused by Naegleria fowleri, occurs mostly in children and young adults. Patients present with abrupt fever, headache, nausea and vomiting, disturbances in smell and taste, meningismus, and decreased mental status a few days to 2 weeks after exposure. N fowleri is found in warm fresh water and moist soil. Infection is often associated with swimming in warm freshwater lakes and using contaminated tap water for sinus irrigation. CNS invasion occurs after nasal inoculation of N fowleri which travel along the olfactory nerves via the cribiform plate to the brain. The disease is rapidly progressive and nearly universally fatal within a week of symptom onset.
Chronic granulomatous encephalitis, caused by Acanthamoeba or Balamuthia, can occur in immunocompotent patients, but occurs more commonly in immunocompromised patients. There is no association with freshwater swimming. This disease has an insidious onset of focal neurologic deficits, and approximately 50% of patients present with headache. Skin, sinus, or lung infections with Acanthamoeba precede many of the CNS infections and may still be present at the onset of neurologic disease. The granulomatous encephalitis progresses to fatal outcome over a period of weeks to months (average 6 weeks).
Acanthamoeba keratitis is a corneal infection associated with minor trauma or use of soft contact lenses in otherwise healthy persons. Clinical findings of Acanthamoeba keratitis include radial keratoneuritis and stromal ring infiltrate. Amebic keratitis usually follows an indolent course and initially may resemble herpes simplex or bacterial keratitis; delay in diagnosis is associated with worse outcomes.
 Clinical Findings & Differential Diagnosis
Clinical Findings & Differential Diagnosis
Amebic encephalitis should be included in the differential diagnosis of acute meningoencephalitis in children with a history of recent freshwater swimming. The CSF is usually hemorrhagic, with leukocyte counts that may be normal early in the disease but later range from 400 to 2600/mL with neutrophil predominance, low to normal glucose, and elevated protein. The etiologic diagnosis relies on finding trophozoites on a wet mount of the CSF. Immunofluorescent and PCR-based diagnostic assays are available through the CDC.
Granulomatous encephalitis is diagnosed by brain biopsy of CT-identified nonenhancing lucent areas. The CSF of these patients is usually nondiagnostic with a lymphocytic pleocytosis, mild to severe elevation of protein (> 1000 mg/dL), and normal or low glucose. Acanthamoeba and Balamuthia amoebas have only rarely been found in the CSF; however, they can be visualized in brain biopsies or grown from brain or other infected tissues. Immunofluorescent and PCR-based diagnostic assays are available through the CDC.
Acanthamoeba keratitis is diagnosed by finding the trophozoites in corneal scrapings or by isolating the parasite from corneal specimens or contact lens cultures.
 Prevention
Prevention
Because primary amebic meningitis occurs infrequently, active surveillance of lakes for N fowleri is not warranted. However, in the presence of a documented case, it is advisable to close the implicated lake to swimming. Sterile or boiled water should be used for sinus irrigation. Acanthamoeba keratitis can be prevented by heat disinfection of contact lenses, by storage of lenses in sterile solutions, and by not wearing lenses when swimming in freshwater or showering.
 Treatment
Treatment
Treatment of acute amebic meningoencephalitis caused by N fowleri should be attempted with high-dose intravenous amphotericin B with the possible addition of miconazole and rifampin. Azithromycin has in vitro and in vivo activity against Naeglaria and may be tried as an adjuvant. Successful treatment of Balamuthia encephalitis has been reported using a combination of flucytosine, pentamidine, fluconazole, sulfadiazine, and a macrolide. Acanthamoeba encephalitis should be treated with a combination of pentamidine, an azole compound, flucytosine, and sulfadiazine.
Acanthamoeba keratitis responds well to surgical debridement followed by 3–4 weeks of topical 1% miconazole; 0.1% propamidine isethionate; and polymyxin B sulfate, neomycin, and bacitracin (Neosporin).
http://www.cdc.gov/parasites/naegleria/.
http://www.cdc.gov/parasites/balamuthia/.
http://www.cdc.gov/parasites/acantamoeba/.
Visvesvara GS: Amebic meningoencephalitides: challenges in diagnosis and treatment. Curr Opin Infect Dis 2010;23(6):590–594 [PMID: 20802332].
Yoder JS et al. Primary Amebic Encephalitis Deaths Associated with Sinus Irrigation Using Contaminated Tap Water. Clin Infect Dis 2012;55(9):e79–e85 [PMID: 22919000].
TRICHOMONIASIS
Trichomonas vaginalis infection is discussed in Chapter 44.