Chapter 103 Infections of the Neonatal Infant
103.1 Pathogenesis and Epidemiology
Bizzarro MJ, Dembry LM, Baltimore RS, et al. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689-696.
Bizzarro MJ, Raskind C, Baltimore RS, et al. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. 2005;116:595-602.
Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1384.
Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16:235-244.
Vergnano S, Sharland M, Kasembe P, et al. Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed. 2005;90:F220-F224.
Zaidi AKM, Thaver D, Asas S, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J. 2009;28:S10-S18.
103.2 Modes of Transmission and Pathogenesis
Pathogenesis of Intrauterine Infection
Intrauterine infection is a result of clinical or subclinical maternal infection with a variety of agents (cytomegalovirus [CMV], Treponema pallidum, Toxoplasma gondii, rubella virus, varicella virus, parvovirus B19) and hematogenous transplacental transmission to the fetus. Transplacental infection may occur at any time during gestation, and signs and symptoms may be present at birth or may be delayed for months or years (Fig. 103-1). Infection may result in early spontaneous abortion, congenital malformation, intrauterine growth restriction, premature birth, stillbirth, acute or delayed disease in the neonatal period, or asymptomatic persistent infection with sequelae later in life. In some cases, no apparent effects are seen in the newborn infant.
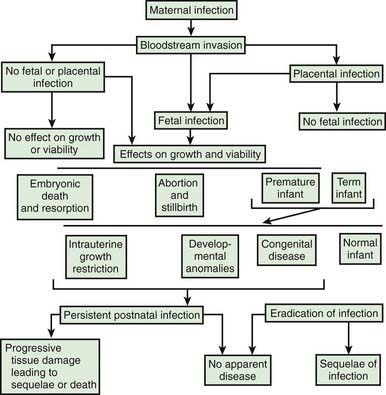
Figure 103-1 Pathogenesis of hematogenous transplacental infections.
(From Klein JO, Remington JS: Current concepts of infections of the fetus and newborn infant. In Remington JS, Klein JO, editors: Infectious diseases of the fetus and newborn infant, ed 5, Philadelphia, 2002, WB Saunders.)
The timing of infection during gestation affects the outcome. First-trimester infection may alter embryogenesis, with resulting congenital malformations (congenital rubella) (Chapter 239). Third-trimester infection often results in active infection at the time of delivery (toxoplasmosis, syphilis) (Chapters 282 and 210). Infections that occur late in gestation may lead to a delay in clinical manifestations until some time after birth (syphilis).
Maternal infection is a necessary prerequisite for transplacental infection. For some etiologic agents (rubella), maternal immunity is effective and antibody is protective for the fetus. For other agents (CMV), maternal antibody may ameliorate the outcome of infection or may have no effect (Chapter 247). Even without maternal antibody, transplacental transmission of infection to a fetus is variable because the placenta may function as an effective barrier.
Pathogenesis of Ascending Bacterial Infection
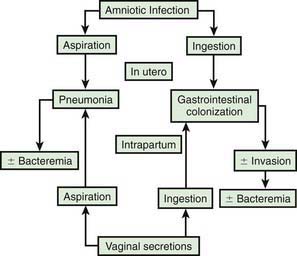
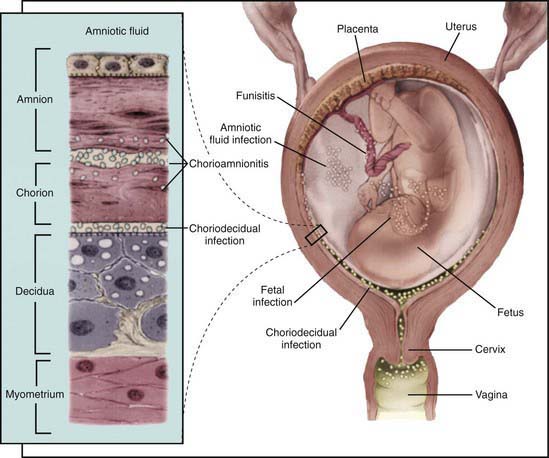
In most cases, the fetus or neonate is not exposed to potentially pathogenic bacteria until the membranes rupture and the infant passes through the birth canal and/or enters the extrauterine environment. The human birth canal is colonized with aerobic and anaerobic organisms that may result in ascending amniotic infection and/or colonization of the neonate at birth. Vertical transmission of bacterial agents that infect the amniotic fluid and/or vaginal canal may occur in utero or, more commonly, during labor and/or delivery (Fig. 103-2). Chorioamnionitis results from microbial invasion of amniotic fluid, often as a result of prolonged rupture of the chorioamniotic membrane. Amniotic infection may also occur with apparently intact membranes or with a relatively brief duration of membrane rupture. The term chorioamnionitis refers to the clinical syndrome of intrauterine infection, which includes maternal fever, with or without local or systemic signs of chorioamnionitis (uterine tenderness, foul-smelling vaginal discharge/amniotic fluid, maternal leukocytosis, maternal and/or fetal tachycardia). Chorioamnionitis may also be asymptomatic, diagnosed only by amniotic fluid analysis or pathologic examination of the placenta. The rate of histologic chorioamnionitis is inversely related to gestational age at birth (Fig. 103-3) and directly related to duration of membrane rupture. Rupture of membranes for longer than 24 hr was once considered prolonged because microscopic evidence of inflammation of the membranes is uniformly present when the duration of rupture exceeds 24 hr. At 18 hr of membrane rupture, however, the incidence of early-onset disease with group B streptococcus (GBS) increases significantly; 18 hr is the appropriate cutoff for increased risk of neonatal infection.
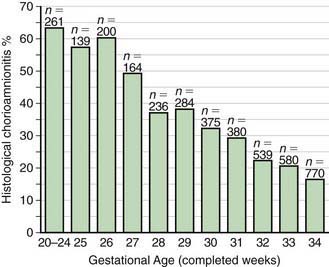
Figure 103-3 Histologic chorioamnionitis in liveborn preterm babies by gestational age (n = 3,928 babies).
(From Lahra MM, Jeffery HE: A fetal response to chorioamnionitis is associated with early survival after preterm birth, Am J Obstet Gynecol 190:147–151, 2004.)
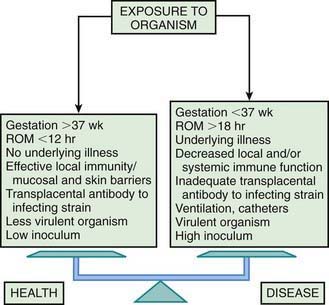
Bacterial colonization does not always result in disease. Factors influencing which colonized infant will experience disease are not well understood but include prematurity, underlying illness, invasive procedures, inoculum size, virulence of the infecting organism, genetic predisposition, the innate immune system, host response, and transplacental maternal antibodies (Fig. 103-4). Aspiration or ingestion of bacteria in amniotic fluid may lead to congenital pneumonia or systemic infection, with manifestations becoming apparent before delivery (fetal distress, tachycardia), at delivery (failure to breathe, respiratory distress, shock), or after a latent period of a few hours (respiratory distress, shock). Aspiration or ingestion of bacteria during the birth process may lead to infection after an interval of 1-2 days.
Mylonakis E, Paliou M, Hohmann EL, et al. Listeriosis during pregnancy. Medicine (Baltimore). 2002;81:260-269.
Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009;63:862-867.
Phonesamart W, Yoksan S, Vanaprapa N, et al. Dengue virus infection in late pregnancy and transmission to the infants. Pediatr Infect Dis J. 2008;27:500-504.
Ramful D, Carbonnier M, Pasquet M, et al. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007;26:811-815.
Read JS, Cannom MJ, Stanberry LR, et al. Prevention of mother-to-child transmission of viral infections. Curr Prob Pediatr Adolesc Health Care. 2008;38:269-302.
103.3 Immunity
Natural Killer Cells
Natural killer (NK) cells are a subgroup of lymphocytes that are cytolytic against cells infected with viruses. NK cells also lyse cells coated with antibody in a process called antibody-dependent cell-mediated cytotoxicity (ADCC). NK cells appear early in gestation and are present in cord blood in numbers equivalent to those in adults; neonatal NK cells have decreased cytotoxic activity and ADCC in comparison with adult cells. The diminished cytotoxicity against herpes simplex virus (HSV)–infected cells may predispose to disseminated HSV infection in newborns (Chapter 249).
103.4 Etiology of Fetal and Neonatal Infection
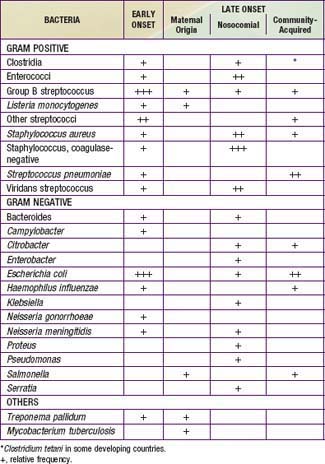
A number of agents may infect newborns in utero, intrapartum, or postpartum (Tables 103-1 and 103-2). Intrauterine transplacental infections of significance to the fetus and/or newborn include syphilis, rubella, CMV, toxoplasmosis, parvovirus B19, and varicella. Although HSV, HIV, hepatitis B virus (HBV), hepatitis C virus, and tuberculosis (TB) can each result in transplacental infection, the most common mode of transmission for these agents is intrapartum, during labor and delivery with passage through an infected birth canal (HIV, HSV, HBV), or postpartum, from contact with an infected mother or caretaker (TB) or with infected breast milk (HIV).
Congenital pneumonia may be caused by CMV, rubella virus, and T. pallidum and, less commonly, by the other agents producing transplacental infection (Table 103-3). Microorganisms causing pneumonia acquired during labor and delivery include GBS, gram-negative enteric aerobes, Listeria monocytogenes, genital Mycoplasma, Chlamydia trachomatis, CMV, HSV, and Candida species.
Table 103-3 ETIOLOGIC AGENTS OF NEONATAL PNEUMONIA ACCORDING TO TIMING OF ACQUISITION
TRANSPLACENTAL
PERINATAL
POSTNATAL
* More likely with mechanical ventilation or indwelling catheters, or after abdominal surgery.
Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154:842-848.
DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
Mullany LC, Faillace S, Tielsch JM, et al. Incidence and risk factors for newborn umbilical cord infections on Pemba Island, Zanzibar, Tanzania. Pediatr Infect Dis J. 2009;28:503-509.
Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing sepsis in very low birthweight infants. N Engl J Med. 2002;347:240-247.
Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817-826.
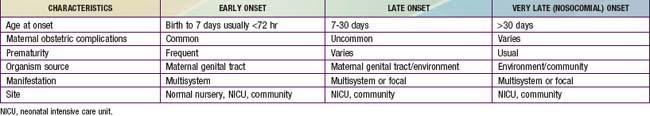
103.5 Epidemiology of Early- and Late-Onset Neonatal Infections
The terms early-onset infection and late-onset infection refer to the different ages at onset of infection in the neonatal period (Table 103-4). Although these disorders were originally divided arbitrarily into infections occurring before and after 1 wk of life, it is more useful to separate early- and late-onset infections according to peripartum pathogenesis. Early-onset infections are acquired before or during delivery (vertical mother-to-child transmission). Late-onset infections develop after delivery from organisms acquired in the hospital or the community. The age at onset depends on the timing of exposure and virulence of the infecting organism. Very-late-onset infections (onset after 1 mo of life) may also occur, particularly in VLBW preterm infants or term infants requiring prolonged neonatal intensive care.
A study from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network documented rates of early-onset sepsis among approximately 200,000 live births at Network centers. The overall rate of early-onset sepsis was 1.2 cases per 1000 live births with rates inversely related to birthweight (401-1500 g BW, 12.33/1000; 1501-2500 g BW, 1.96/1000; >2500 g BW, 0.71/1000) (Table 103-5).
Table 103-5 RATES OF EARLY-ONSET SEPSIS PER 1,000 LIVE BIRTHS: NICHD NEONATAL RESEARCH NETWORK/CDC SURVEILLANCE STUDY OF EARLY-ONSET SEPSIS

Prematurity
The most important neonatal factor predisposing to infection is prematurity or LBW. Preterm LBW infants have a 3- to 10-fold higher incidence of infection than full-term normal birthweight infants. Possible explanations are as follows: (1) maternal genital tract infection is considered to be an important cause of preterm labor, with an increased risk of vertical transmission to the newborn (Figs. 103-5 and 103-6); (2) the frequency of intra-amniotic infection is inversely related to gestational age (see Fig. 103-3); (3) premature infants have documented immune dysfunction; and (4) premature infants often require prolonged intravenous access, endotracheal intubation, or other invasive procedures that provide a portal of entry or impair barrier and clearance mechanisms.
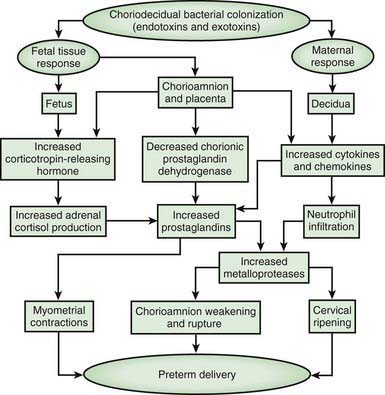
Figure 103-5 Potential pathways from choriodecidual bacterial colonization to preterm delivery.
(From Goldenberg RL, Hauth JA, Andrews WW: Intrauterine infection and preterm delivery, N Engl J Med 342:1500–1507, 2000. Copyright 2000, Massachusetts Medical Society.)
Nosocomial Infections
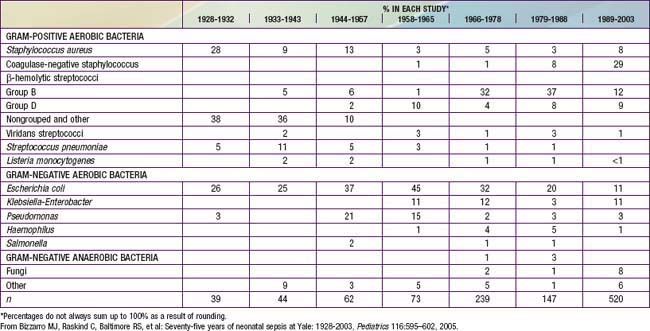
Coagulase-negative staphylococci are the most frequent neonatal nosocomial pathogens. In a cohort of 6,215 VLBW infants in the NICHD Neonatal Research Network, gram-positive agents were associated with 70%, gram-negative with 18%, and fungi with 12% of cases of late-onset sepsis (Table 103-6). Coagulase-negative staphylococcus, the single most common organism, was isolated in 48% of these infections. The emergence of nosocomial bacterial pathogens resistant to multiple antibiotics is a growing concern. Among NICU patients, methicillin-resistant S. aureus, vancomycin-resistant enterococci, and multidrug-resistant gram-negative pathogens are particularly alarming. Organisms responsible for all categories of neonatal sepsis and meningitis may change with time (Table 103-7).
Table 103-6 DISTRIBUTION OF PATHOGENS ASSOCIATED WITH THE 1ST EPISODE OF LATE-ONSET SEPSIS IN VERY LOW BIRTHWEIGHT INFANTS*
| ORGANISM† | N | % |
|---|---|---|
| Gram-positive organisms: | 922 | 70.2 |
| Staphylococcus—coagulase negative | 629 | 47.9 |
| Staphylococcus aureus | 103 | 7.8 |
| Enterococcus spp. | 43 | 3.3 |
| Group B streptococci | 30 | 2.3 |
| Other | 117 | 8.9 |
| Gram-negative organisms: | 231 | 17.6 |
| Escherichia coli | 64 | 4.9 |
| Klebsiella | 52 | 4.0 |
| Pseudomonas | 35 | 2.7 |
| Enterobacter | 33 | 2.5 |
| Serratia | 29 | 2.2 |
| Other | 18 | 1.4 |
| Fungi: | 160 | 12.2 |
| Candida albicans | 76 | 5.8 |
| Candida parapsilosis | 54 | 4.1 |
| Other | 30 | 2.3 |
| TOTAL | 1,313 | 100 |
* National Institute of Child Health and Human Development Neonatal Research Network, September 1, 1998, through August 31, 2000.
† Patients with dual infections and patients with presumed coagulase-negative staphylococci (CONS) contaminants excluded. According to the definitions in text, 276 (44%) CONS were definite infections and 353 (56%) were possible infections.
From Stoll BJ, Hansen N, Fanaroff AA, et al: Late-onset sepsis in very low birthweight neonates: the experience of the NICHD Neonatal Research Network, Pediatrics 110:285–291, 2002.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree