 General Considerations
General Considerations
Group A streptococci (GAS) are common gram-positive bacteria producing a wide variety of clinical illnesses, including acute pharyngitis, impetigo, cellulitis, and scarlet fever, the generalized illness caused by strains that elaborate erythrogenic toxin. GAS can also cause pneumonia, septic arthritis, osteomyelitis, meningitis, and other less common infections. GAS infections may also produce nonsuppurative sequelae (rheumatic fever and acute glomerulonephritis).
The cell walls of streptococci contain both carbohydrate and protein antigens. The C-carbohydrate antigen determines the group, and the M- or T-protein antigens determine the specific type. In most strains, the M protein appears to confer virulence, and antibodies developed against the M protein are protective against reinfection with that type.
Almost all GAS are β-hemolytic. These organisms may be carried without symptoms on the skin and in the pharynx, rectum, and vagina. All GAS are sensitive to penicillin. Resistance to erythromycin is common in some countries and has increased in the United States.
 Prevention
Prevention
GAS pharyngitis usually occurs after contact with respiratory secretions of a person infected with GAS. Crowding facilitates spread of GAS and outbreaks of pharyngitis and impetigo can be seen. Prompt recognition and institution of antibiotics may decrease spread. Treatment with antibiotics prevents acute rheumatic fever.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
1. Respiratory infections
A. INFANCY AND EARLY CHILDHOOD (AGE < 3 YEARS)—The onset is insidious, with mild symptoms (low-grade fever, serous nasal discharge, and pallor). Otitis media is common. Exudative pharyngitis and cervical adenitis are uncommon in this age group.
B. CHILDHOOD TYPE—Onset is sudden, with fever and marked malaise and often with repeated vomiting. The pharynx is sore and edematous, and generally there is tonsillar exudate. Anterior cervical lymph nodes are tender and enlarged. Small petechiae are frequently seen on the soft palate. In scarlet fever, the skin is diffusely erythematous and appears sunburned and roughened (sandpaper rash). The rash is most intense in the axillae, groin, and on the abdomen and trunk. It blanches except in the skin folds, which do not blanch and are pigmented (Pastia sign). The rash usually appears 24 hours after the onset of fever and rapidly spreads over the next 1–2 days. Desquamation begins on the face at the end of the first week and becomes generalized by the third week. Early in the course of infection, there is circumoral pallor and the surface of the tongue is coated white, with the papillae enlarged and bright red (white strawberry tongue). Subsequently desquamation occurs, and the tongue appears beefy red (strawberry tongue). Petechiae may be seen on all mucosal surfaces.
C. ADULT TYPE—The adult type of GAS is characterized by exudative or nonexudative tonsillitis with fewer systemic symptoms, lower fever, and no vomiting. Scarlet fever is uncommon in adults.
2. Impetigo—Streptococcal impetigo begins as a papule that vesiculates and then breaks, leaving a denuded area covered by a honey-colored crust. Both Staphylococcus aureus and GAS are isolated in some cases. The lesions spread readily and diffusely. Local lymph nodes may become swollen and inflamed. Although the child often lacks systemic symptoms, a high fever and toxicity may be present. If flaccid bullae are noted, the disease is called bullous impetigo and is caused by an epidermolytic toxin-producing strain of S aureus.
3. Cellulitis—The portal of entry is often an insect bite or superficial abrasion. A diffuse, rapidly spreading cellulitis occurs that involves the subcutaneous tissues and extends along the lymphatic pathways with only minimal local suppuration. Local acute lymphadenitis occurs. The child is usually acutely ill, with fever and malaise. In classic erysipelas, the involved area is bright red, swollen, warm, and very tender. The infection may extend rapidly from the lymphatics to the bloodstream.
Streptococcal perianal cellulitis is an entity peculiar to young children. Pain with defecation often leads to constipation, which may be the presenting complaint. The child is afebrile and otherwise well. Perianal erythema, tenderness, and painful rectal examination are the only abnormal physical findings. Scant rectal bleeding with defecation may occur. A perianal swab culture usually yields heavy growth of GAS. A variant of this syndrome is streptococcal vaginitis in prepubertal girls. Symptoms are dysuria and pain; marked erythema and tenderness of the introitus and blood-tinged discharge are seen.
4. Necrotizing fasciitis—This dangerous disease is reported sporadically and may occur as a complication of varicella infection. About 20%–40% of cases are due to GAS; 30%–40% are due to S aureus; and the rest are a result of mixed bacterial infections. The disease is characterized by extensive necrosis of superficial fasciae, undermining of surrounding tissue, and usually systemic toxicity. Initially the skin overlying the infection is tender and pale red without distinct borders, resembling cellulitis. Blisters or bullae may appear. The color deepens to a distinct purple or in some cases becomes pale. Tenderness out of proportion to the clinical appearance, skin anesthesia (due to infarction of superficial nerves), or “woody” induration suggest necrotizing fasciitis. Involved areas may develop mild to massive edema. Early recognition and aggressive debridement of necrotic tissue are essential.
5. Group A streptococcal infections in newborn nurseries—GAS epidemics occur occasionally in nurseries. The organism may be introduced into the nursery from the vaginal tract of a mother or from the throat or nose of a mother or a staff member. The organism then spreads from infant to infant. The umbilical stump is colonized while the infant is in the nursery. Like staphylococcal infections, there may be no or few clinical manifestations while the infant is still in the nursery. Most often, a colonized infant develops a chronic oozing omphalitis days later. The organism may spread from the infant to other family members. Serious and even fatal infections may develop, including sepsis, meningitis, empyema, septic arthritis, and peritonitis.
6. Streptococcal sepsis—Serious illness from GAS sepsis is now more common both in children and in adults. Rash and scarlet fever may be present. Prostration and shock result in high mortality rates. Pharyngitis is uncommon as an antecedent illness. Underlying disease is a predisposing factor.
7. Streptococcal toxic shock syndrome (STSS)—Toxic shock syndrome caused by GAS has been defined. Like S aureus–associated toxic shock, multiorgan system involvement is a prominent part of the illness. The diagnostic criteria include (1) isolation of GAS from a normally sterile site, (2) hypotension or shock, and (3) at least two of the following: renal impairment (creatinine > two times the upper limit of normal for age), thrombocytopenia (< 100,000/mm3), or coagulopathy, liver involvement (transaminases > two times normal), acute respiratory distress syndrome, generalized erythematous macular rash or soft tissue necrosis (myositis, necrotizing fasciitis, gangrene). In cases that otherwise meet clinical criteria, isolation of GAS from a nonsterile site (throat, wound, or vagina) is indicative of a probable cause.
B. Laboratory Findings
Leukocytosis with a marked shift to the left is seen early. Eosinophilia regularly appears during convalescence. β-Hemolytic streptococci are cultured from the throat or site of infection. For suspected GAS pharyngitis, the throat should be swabbed and the specimen sent for GAS testing (rapid antigen detection tests and/or culture for GAS) because the clinical features of some viral infections may overlap with the clinical features of GAS. In children and adolescents, negative rapid antigen tests should be backed up by a culture. Patients with positive rapid strep antigen tests do not need a confirmation by throat culture, since the specificities of antigen tests are high. The organism may be cultured from the skin and by needle aspiration from subcutaneous tissues and other involved sites such as infected nodes. Occasionally blood cultures are positive.
Antistreptolysin O (ASO) titers rise about 150 units within 2 weeks after acute infection. Elevated ASO and anti-DNase B titers are useful in documenting prior throat infections in cases of acute rheumatic fever. The streptozyme test detects antibodies to streptolysin O, hyaluronidase, streptokinase, DNase B, and NADase. It is somewhat more sensitive than the measurement of ASO titers.
Proteinuria, cylindruria, and minimal hematuria may be seen early in children with streptococcal infection. True poststreptococcal glomerulonephritis is seen 1–4 weeks after the respiratory or skin infection.
 Differential Diagnosis
Differential Diagnosis
Streptococcal infection in early childhood must be differentiated from adenovirus and other respiratory virus infections. The pharyngitis in herpangina (coxsackievirus A) is vesicular or ulcerative. Herpes simplex also causes ulcerative lesions, which most commonly involve the anterior pharynx, tongue, and gums. In infectious mononucleosis, the pharyngitis is also exudative, but splenomegaly and generalized adenopathy are typical, and laboratory findings are often diagnostic (atypical lymphocytes, elevated liver enzymes, and a positive heterophile or other serologic test for mononucleosis). Uncomplicated streptococcal pharyngitis improves within 24–48 hours if penicillin is given and by 72–96 hours without antimicrobials.
Group G and group C streptococci are uncommon causes of pharyngitis but have been implicated in epidemics of sore throat in college students. Acute rheumatic fever does not occur following group G or group C infection, although acute glomerulonephritis (AGN) is a complication. Arcanobacterium hemolyticum may cause pharyngitis with scarlatina-like or maculopapular truncal rash. In diphtheria, systemic symptoms, vomiting, and fever are less marked; pharyngeal pseudomembrane is confluent and adherent; the throat is less red; and cervical adenopathy is prominent. Pharyngeal tularemia causes white rather than yellow exudate. There is little erythema, and cultures for β-hemolytic streptococci are negative. A history of exposure to rabbits and a failure to respond to antimicrobials may suggest the diagnosis. Leukemia and agranulocytosis may present with pharyngitis and are diagnosed by bone marrow examination.
Scarlet fever must be differentiated from other exanthematous diseases (principally rubella), erythema due to sunburn, drug reactions, Kawasaki disease, toxic shock syndrome (TSS), and staphylococcal scalded skin syndrome (see also Table 40–3).
 Complications
Complications
Suppurative complications of GAS infections include sinusitis, otitis, mastoiditis, cervical lymphadenitis, pneumonia, empyema, septic arthritis, and meningitis. Spread of streptococcal infection from the throat to other sites—principally the skin (impetigo) and vagina—is common and should be considered in every instance of chronic vaginal discharge or chronic skin infection, such as that complicating childhood eczema. Both acute rheumatic fever and AGN are nonsuppurative complications of GAS infections.
A. Acute Rheumatic Fever (See Chapter 20)
B. Acute Glomerulonephritis
AGN can follow streptococcal infections of either the pharynx or the skin—in contrast to rheumatic fever, which follows pharyngeal infection only. AGN may occur at any age, even infancy. In most reports of AGN, males predominate by a ratio of 2:1. Rheumatic fever occurs with equal frequency in both sexes. Certain M types are associated strongly with poststreptococcal glomerulonephritis (nephritogenic types). The serotypes producing disease on the skin often differ from those found in the pharynx.
The incidence of AGN after streptococcal infection is variable and has ranged from 0% to 28%. Several outbreaks of AGN in families have involved 50%–75% of siblings of affected patients in 1- to 7-week periods. Second attacks of glomerulonephritis are rare. The median period between infection and the development of glomerulonephritis is 10 days. In contrast, acute rheumatic fever occurs after a median of 18 days.
C. Poststreptococcal Reactive Arthritis
Following an episode of group A streptococcal pharyngitis, a reactive arthritis develops in some patients. This reactive arthritis is believed to be due to immune complex deposition and is seen about 1–2 weeks following the acute infection. Patients with poststreptococcal reactive arthritis do not have the full constellation of clinical and laboratory criteria needed to fulfill the Jones criteria for a diagnosis of acute rheumatic fever.
 Treatment
Treatment
A. Specific Measures
Treatment is directed toward both eradication of acute infection and prevention of rheumatic fever. In patients with pharyngitis, antibiotics should be started early to relieve symptoms and should be continued for 10 days to prevent rheumatic fever. Although early therapy has not been shown to prevent AGN, it seems advisable to treat impetigo promptly in sibling contacts of patients with poststreptococcal nephritis. Neither sulfonamides nor trimethoprim-sulfamethoxazole (TMP-SMX) is effective in the treatment of streptococcal infections. Although topical therapy for impetigo with antimicrobial ointments (especially mupirocin) is as effective as systemic therapy, it does not eradicate pharyngeal carriage and is less practical for extensive disease.
1. Penicillin—For GAS pharyngitis, the following regimens can be used. Except for penicillin-allergic patients, penicillin V (phenoxymethyl penicillin) is the drug of choice. Penicillin resistance has never been documented. For children weighing less than 27 kg, the regimen is 250 mg, given orally two or three times a day for 10 days. For heavier children, adolescents, or adults 500 mg two or three times a day is recommended. Giving penicillin V twice daily is as effective as more frequent oral administration or intramuscular therapy. Once-daily oral amoxicillin (50 mg/kg, maximum 1000 mg) has been shown to be as effective as penicillin V given three times a day. Another alternative for treatment of pharyngitis and impetigo is a single dose of benzathine penicillin G, given intramuscularly (600,000 units for children weighing < 60 lb [27.2 kg] and 1.2 million units for children weighing > 60 lb [27.2 kg]). Intramuscular delivery ensures compliance, but is painful. Parenteral therapy is indicated if vomiting or sepsis is present. Mild cellulitis due to GAS may be treated orally or intramuscularly.
Cellulitis requiring hospitalization can be treated with aqueous penicillin G (150,000 U/kg/d, given intravenously in four to six divided doses) or cefazolin (100 mg/kg/d, given intravenously in three divided doses) until there is marked improvement. Penicillin V (50 mg/kg/d in four divided doses) or cephalexin (50–75 mg/kg/d in four divided doses) may then be given orally to complete a 10-day course. Acute cervical lymphadenitis may require incision and drainage. Treatment of necrotizing fasciitis requires emergency surgical debridement followed by high-dose parenteral antibiotics appropriate to the organisms cultured.
2. Other antibiotics—Cephalexin and cefadroxil are other effective oral antimicrobials. For penicillin-allergic patients with pharyngitis or impetigo the following alternative regimens have been used: azithromycin (12 mg/kg/d; maximum 500 mg per dose) once daily for 5 days, or clindamycin (20–30 mg/kg/d in three divided doses; maximum 300 mg per dose) for 10 days. Patients with immediate, anaphylactic hypersensitivity to penicillin should not receive cephalosporins, because up to 15% will also be allergic to cephalosporins. Macrolide resistance rates vary and may be high in some areas of the world. In general, macrolide resistance rates in most areas of the US are between 5% and 8%. If macrolide resistance rates are known to be high in a given region, an alternative agent can be selected. In most studies, bacteriologic failures after cephalosporin therapy are less frequent than failures following penicillin. However, there are few conclusive data on the ability of these agents to prevent rheumatic fever. Therefore, penicillin remains the agent of choice for nonallergic patients. Many strains are resistant to tetracycline.
For infections requiring intravenous therapy, aqueous penicillin G (250,000 U/kg in six divided doses) given intravenously is usually the drug of choice. Cefazolin (100 mg/kg/d intravenously or intramuscularly in three divided doses); clindamycin (30–40 mg/kg/d intravenously in four divided doses); and vancomycin (40 mg/kg/d intravenously in four divided doses) are alternatives in penicillin-allergic patients. Clindamycin should not be used alone empirically for severe, suspected GAS infections because a small percentage of isolates in the United States are resistant to it. Some physicians use both penicillin and clindamycin in patients with necrotizing fasciitis or STSS.
3. Serious GAS disease—Serious GAS infections, such as pneumonia, osteomyelitis, septic arthritis, sepsis, endocarditis, meningitis, and STSS, require parenteral antimicrobial therapy. Penicillin G is the drug of choice for these invasive infections. Clindamycin, in addition to penicillin G, is advocated by many experts for STSS or necrotizing fasciitis. Necrotizing fasciitis requires prompt surgical debridement. In STSS, volume status and blood pressure should be monitored and patients evaluated for a focus of infection, if not readily apparent. Intravenous immune globulin (in addition to antibiotics) has been used in severe cases.
4. Treatment failure—Even when compliance is perfect, organisms will be found in cultures in 5%–35% of children after cessation of therapy. Reculture is indicated only in patients with relapse or recrudescence of pharyngitis or those with a personal or family history of rheumatic fever. Repeat treatment at least once with an oral cephalosporin or clindamycin is indicated in patients with recurrent culture-positive pharyngitis.
5. Prevention of recurrences in rheumatic individuals—The preferred prophylaxis for rheumatic individuals is benzathine penicillin G, 1.2 million units (600,000 units for patients weighing less than 27 kg) intramuscularly every 4 weeks. If the risk of streptococcal exposure is high, every-3-week dosing is preferred. One of the following alternative oral prophylactic regimens may be used: penicillin V, 250 mg twice daily; or sulfadiazine, 0.5 g once a day (if < 27 kg) or 1 g once a day (if > 27 kg). In patients allergic to both penicillin and sulfonamide drugs, erythromycin 250 mg twice daily orally can be used. If carditis is absent, continued prophylaxis is recommended for at least 5 years after the last episode of acute rheumatic fever or until 21 years of age (whichever is longer). Prophylaxis should be continued longer if the risk of contact with persons with GAS is high (eg, parents of school-aged children, pediatric nurses, and teachers). In the presence of carditis without residual heart or valvular disease, a minimum of 10 years after the last episode of acute rheumatic fever or until 21 years of age (whichever is longer) is the minimum duration. If the patient has residual valvular heart disease, many recommend lifelong prophylaxis. These patients should be at least 10 years from their last episode of rheumatic disease and at least 40 years of age before considering discontinuation of prophylaxis. Those with severe valvular heart disease or with risk of ongoing exposure to GAS may benefit from lifelong prophylaxis. A similar approach to the prevention of recurrences of glomerulonephritis may be used during childhood when there is a suspicion that repeated streptococcal infections coincide with flare-ups of glomerulonephritis.
6. Poststreptococcal reactive arthritis—In contrast to rheumatic fever, nonsteroidal agents may not dramatically improve joint symptoms. However, like patients with rheumatic fever, some patients with poststreptococcal reactive arthritis have developed carditis several weeks to months after their arthritis symptoms began. Patients should be monitored for development of carditis for the next 1–2 years. Some experts recommend antibiotic prophylaxis of these patients (same prophylaxis regimens as in prevention of recurrences of acute rheumatic fever) for 1–2 years and monitoring for signs of carditis (see recommendations for prevention of recurrences of rheumatic fever, above). If carditis does not develop, prophylaxis could then be discontinued. If carditis develops, the patient should be considered to have acute rheumatic fever and prophylaxis continued as described above.
B. General Measures
Acetaminophen is useful for pain or fever. Local treatment of impetigo may promote earlier healing. Crusts should first be soaked off. Areas beneath the crusts should then be washed with soap daily.
C. Treatment of Complications
Rheumatic fever is best prevented by early and adequate penicillin treatment of the streptococcal infection.
D. Treatment of Carriers
Identification and treatment of GAS carriers is difficult. There are no established clinical or serologic criteria for differentiating carriers from the truly infected. Between 10% and 15% of school-aged children in some studies are asymptomatic pharyngeal carriers of GAS. Streptococcal carriers are individuals who do not mount an immune response to the organism and are therefore believed to be at low risk for nonsuppurative sequelae.
Some children receive multiple courses of antimicrobials, with persistence of GAS in the throat, leading to a “streptococcal neurosis” on the part of families.
In certain circumstances, eradication of carriage may be desirable: (1) when a family member has a history of rheumatic fever; (2) when an episode of STSS or necrotizing fasciitis has occurred in a household contact; (3) multiple, recurring, documented episodes of GAS in family members despite adequate therapy; and (4) during an outbreak of rheumatic fever or GAS-associated glomerulonephritis. Clindamycin (20–30 mg/kg/d, given orally in three divided doses; maximum dose 300 mg) or a combination of rifampin (20 mg/kg/d, given orally for 4 days) and penicillin in standard dosage given orally has been used to attempt eradication of carriage.
 Prognosis
Prognosis
Death is rare except in infants or young children with sepsis or pneumonia. The febrile course is shortened and complications eliminated by early and adequate treatment with penicillin.
Shulman ST et al: Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55(10):e86–e102 [PMID: 22965026].
Tanz RR, Shulman ST: Chronic pharyngeal carriage of group A streptococci. Pediatr Infect Dis J 2007;26:175 [PMID: 17259882].
Wessels MR: Clinical practice: streptococcal pharyngitis. N Engl J Med 2011;364:648–655 [PMID: 21323542].
GROUP B STREPTOCOCCAL INFECTIONS
 Prevention
Prevention
Many women of childbearing age possess type-specific circulating antibody to the polysaccharide antigens for group B Streptococcus (GBS). These antibodies are transferred to the newborn via the placental circulation. GBS carriers delivering healthy infants have significant serum levels of IgG antibody to this antigen. In contrast, women delivering infants who develop either early- or late-onset GBS disease rarely have detectable antibody in their sera.
Monovalent and bivalent vaccines with type II or III polysaccharide antigens have been studied in pregnant women, with 80%–90% of vaccine recipients developing fourfold or greater increases in GBS capsular polysaccharide type-specific IgG. These reports suggest that a multivalent vaccine could be developed and given to pregnant women to prevent many cases of early-onset GBS disease.
The decline in early-onset GBS disease in young infants is attributed to widespread maternal screening for GBS and intrapartum prophylaxis. The Centers for Disease Control and Prevention (CDC) has issued culture-based maternal guidelines for the prevention of early-onset GBS disease, as well as recommendations for intrapartum prophylaxis and management of babies whose mothers received IAP for prevention of GBS or for chorioamnionitis.
 CDC Recommendations for Prevention of Perinatal GBS Disease
CDC Recommendations for Prevention of Perinatal GBS Disease
1. All pregnant women should be screened at 35–37 weeks’ gestation with a vaginal and rectal culture for GBS. Exceptions: Women with known GBS bacteriuria during the current pregnancy or women who have delivered a previous infant with GBS disease do not need screening—all these women need intrapartum prophylaxis—see Table 42–1.
Table 42–1. Indications and nonindications for intrapartum antibiotic prophylaxis to prevent early-onset group B streptococcal (GBS) disease.
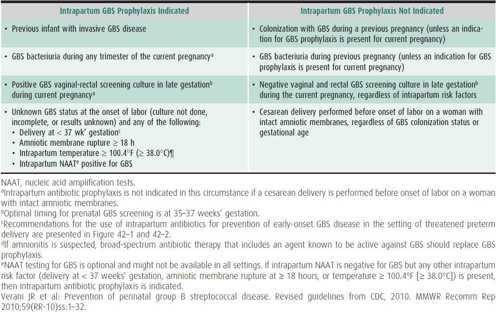
2. Indications and nonindications for intrapartum antibiotic prophylaxis (IAP) to prevent early-onset group B streptococcal (GBS) disease—see Table 42–1.
3. Algorithm for screening for GBS colonization and use of intrapartum prophylaxis for women with preterm labor (PTL)—see Figure 42–1.
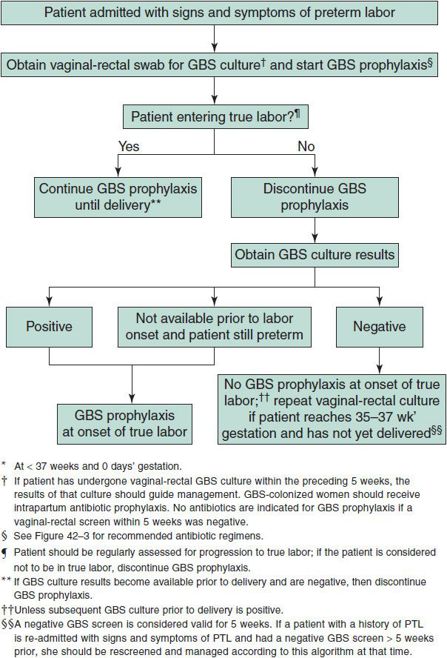
 Figure 42–1. Algorithm for screening for group B streptococcal (GBS) colonization and use of intrapartum prophylaxis for women with preterm* labor (PTL). (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
Figure 42–1. Algorithm for screening for group B streptococcal (GBS) colonization and use of intrapartum prophylaxis for women with preterm* labor (PTL). (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
4. Algorithm for screening for GBS colonization and use of intrapartum prophylaxis for women with preterm premature rupture of membranes (pPROM)—see Figure 42–2.
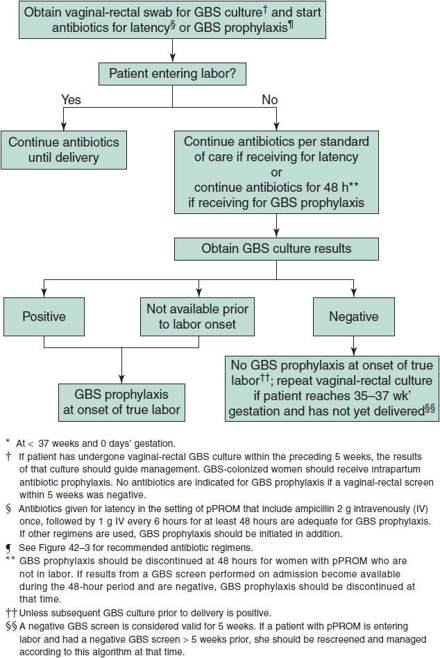
 Figure 42–2. Algorithm for screening for group B streptococcal (GBS) colonization and use of intrapartum prophylaxis for women with preterm* premature rupture of membranes (pPROM). (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
Figure 42–2. Algorithm for screening for group B streptococcal (GBS) colonization and use of intrapartum prophylaxis for women with preterm* premature rupture of membranes (pPROM). (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
5. Recommended regimens for intrapartum antibiotic prophylaxis for prevention of early-onset GBS disease—see Figure 42–3.

 Figure 42–3. Recommended regimens for intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal (GBS) disease.* (Verani JR, McGee L, Schrag SL: Prevention of perinatal Group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
Figure 42–3. Recommended regimens for intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal (GBS) disease.* (Verani JR, McGee L, Schrag SL: Prevention of perinatal Group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
6. Algorithm for secondary prevention of early-onset group B streptococcal (GBS) disease among newborns (management of a newborn whose mother received IAP for prevention of GBS or suspected chorioamnionitis)—see Figure 42–4.
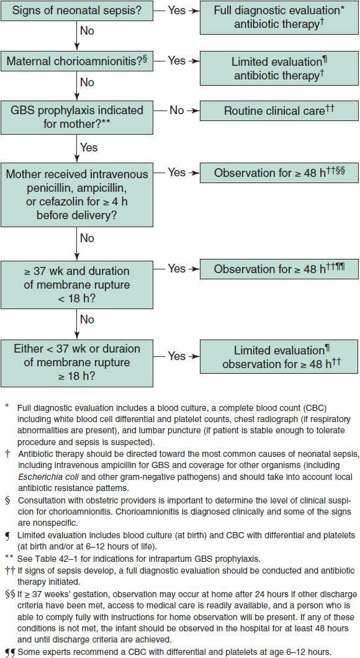
 Figure 42–4. Algorithm for secondary prevention of early-onset group B streptococcal (GBS) disease among newborns. (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
Figure 42–4. Algorithm for secondary prevention of early-onset group B streptococcal (GBS) disease among newborns. (Verani JR, McGee L, Schrag SL: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–32).
 Clinical Findings
Clinical Findings
The incidence of perinatal GBS disease has declined dramatically since screening of pregnant mothers and provision of intrapartum chemoprophylaxis began. Although most patients with GBS disease are infants younger than age 3 months, cases are seen in infants aged 4–5 months. Serious infection also occurs in women with puerperal sepsis, immunocompromised patients, patients with cirrhosis and spontaneous peritonitis, and diabetic patients with cellulitis. Two distinct clinical syndromes distinguished by differing perinatal events, age at onset, and serotype of the infecting strain occur in infants.
Risk factors for early-onset group GBS disease include maternal GBS colonization, gestational age less than 37 weeks, rupture of membranes > 18 hours prior to presentation, young maternal age, history of a previous infant with invasive GBS disease, African-American or Hispanic ethnic origin, and low or absent maternal GBS anticapsular antibodies.
A. Early-Onset Infection
“Early-onset” illness is observed in newborns younger than 7 days old. The onset of symptoms in the majority of these infants occurs in the first 48 hours of life, and most are ill within 6 hours. Apnea is often the first sign. Sepsis, shock, meningitis, apnea, and pneumonia are the most common clinical presentations. There is a high incidence of associated maternal obstetric complications, especially premature labor and prolonged rupture of the membranes. Newborns with early-onset disease are severely ill at the time of diagnosis, and more than 50% die. Although most infants with early-onset infections are full-term, premature infants are at increased risk for the disease. Newborns with early-onset infection acquire GBS in utero as an ascending infection or during passage through the birth canal. When early-onset infection is complicated by meningitis, more than 80% of the bacterial isolates belong to serotype III. Postmortem examination of infants with early-onset disease usually reveals pulmonary inflammatory infiltrates and hyaline membranes containing large numbers of GBS.
B. Late-Onset Infection
“Late-onset” infection occurs in infants between ages 7 days and 4 months (median age at onset, about 4 weeks). Maternal obstetric complications are not usually associated with late-onset infection. These infants are usually not as ill at the time of diagnosis as those with early-onset disease, and the mortality rate is lower. In recent series, about 37% of patients have meningitis and 46% have sepsis. Septic arthritis and osteomyelitis, meningitis, occult bacteremia, otitis media, ethmoiditis, conjunctivitis, cellulitis (particularly of the face or submandibular area), lymphadenitis, breast abscess, empyema, and impetigo have been described. Strains of GBS possessing the capsular type III polysaccharide antigen are isolated from more than 95% of infants with late-onset disease, regardless of clinical manifestations. The exact mode of transmission of the organisms is not well defined.
C. Laboratory Findings
Culture of GBS from a normally sterile site such as blood, pleural fluid, or CSF provides proof of diagnosis. Frequent false-positive results limit the usefulness of testing for GBS antigen in urine and CSF.
 Treatment
Treatment
Intravenous ampicillin and an aminoglycoside is the initial regimen of choice for newborns with presumptive invasive GBS disease. For neonates 7 days of age or younger with meningitis, the recommended ampicillin dosage is 200–300 mg/kg/d, given intravenously in three divided doses. For infants older than 7 days of age, the recommended ampicillin dosage is 300 mg/kg/d, given intravenously in four divided doses.
Penicillin G can be used alone once GBS is identified and clinical and microbiologic responses have occurred. GBS are less susceptible than other streptococci to penicillin, and high doses are recommended, especially for meningitis. In infants with meningitis, the recommended dosage of penicillin G varies with age: for infants age 7 days or younger, 250,000–450,000 U/kg/d, given intravenously in three divided doses; for infants older than age 7 days, 450,000–500,000 U/kg/d, given intravenously in four divided doses.
A second lumbar puncture after 24–48 hours of therapy is recommended by some experts to assess efficacy. Duration of therapy is 2 weeks for uncomplicated meningitis; at least 4 weeks for osteomyelitis, cerebritis, ventriculitis, or endocarditis; and 10 days for bacteremia. Therapy does not eradicate carriage of the organism.
Although streptococci have been universally susceptible to penicillins, increasing minimum inhibitory concentrations (MICs) have been observed in some isolates. Resistance of isolates to clindamycin and erythromycin has increased significantly worldwide in the past few years.
Centers for Disease Control and Prevention (CDC): Prevention of perinatal group B streptococcal disease revised guidelines from CDC, 2010. MMWR 2010;59(RR-10):1–32. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5910.pdf
STREPTOCOCCAL INFECTIONS WITH ORGANISMS OTHER THAN GROUP A OR B
 General Considerations
General Considerations
Streptococci of groups other than A and B are part of the normal flora of humans and can occasionally cause disease. Group C or group G organisms occasionally produce pharyngitis (with an ASO rise), but without risk of subsequent rheumatic fever. AGN may occasionally occur. Group D streptococci and Enterococcus species are normal inhabitants of the gastrointestinal tract and may produce urinary tract infections, meningitis, and sepsis in the newborn, as well as endocarditis. Nosocomial infections caused by Enterococcus are frequent in neonatal and oncology units and in patients with central venous catheters. Nonhemolytic aerobic streptococci and β-hemolytic streptococci are normal flora of the mouth. They are involved in the production of dental plaque and probably dental caries and are the most common cause of subacute infective endocarditis. Finally, there are numerous anaerobic and microaerophilic streptococci, normal flora of the mouth, skin, and gastrointestinal tract, which alone or in combination with other bacteria may cause sinusitis, dental abscesses, brain abscesses, and intra-abdominal or lung abscesses.
 Prevention
Prevention
Streptococci (other than group A or B) are common normal flora in humans. Some disease caused by these organisms can be prevented by maintaining good oral hygiene. Spread of vancomycin resistant enterococcal strains can be limited by good infection control practices in healthcare environments. Development of resistant strains can also be limited by antimicrobial stewardship. There are no vaccines that prevent infections with these organisms.
 Treatment
Treatment
A. Enterococcal Infections
Enterococcus faecalis and Enterococcus faecium are the two most common and most important strains causing human infections. In general, E faecalis is more susceptible to antibiotics than E faecium, but antibiotic resistance is commonly seen with both species. Invasive enterococcal infections should be treated with ampicillin if the isolate is susceptible or vancomycin in combination with gentamicin. Gentamicin should be discontinued if susceptibility testing demonstrates high-level resistance to gentamicin. Isolates that are resistant to both ampicillin and vancomycin necessitate other therapeutic options.
1. Infections with ampicillin-susceptible enterococci—Lower tract urinary infections can be treated with oral amoxicillin. Pyelonephritis should be treated intravenously with ampicillin and gentamicin (gentamicin dosing may need to be adjusted for altered renal function). Sepsis or meningitis in the newborn should be treated intravenously with a combination of ampicillin and gentamicin. Peak serum gentamicin levels of 3–5 mcg/mL are adequate as gentamicin is used as a synergistic agent. Endocarditis requires 6 weeks of intravenous treatment. Ampicillin or penicillin in combination with gentamicin is used in susceptible strains. Consult the American Heart Association guidelines for infective endocarditis for treatment recommendations for endocarditis.
2. Infections with ampicillin-resistant or vancomycin-resistant enterococci—Ampicillin-resistant enterococci are often susceptible to vancomycin (40–60 mg/kg/d in four divided doses). Vancomycin-resistant enterococci are usually also resistant to ampicillin. Linezolid is approved for use in children only for vancomycin-resistant E faecium infections. Two other agents are approved in adults against certain vancomycin-resistant enterococci. Daptomycin is approved for adults with vancomycin-resistant E faecalis infections, Quinupristin-dalfopristin is approved for adults with vancomycin-resistant E faecium (not effective against E faecalis) infections. Isolates resistant to these newer agents (linezolid, daptomycin, quinupristin-dalfopristin) have been reported. Infectious disease consultation is recommended when use of these drugs is entertained or when vancomycin-resistant enterococcal infections are identified.
B. Viridans Streptococci Infections (Subacute Infective Endocarditis)
It is important to determine the penicillin sensitivity of the infecting strain as early as possible in the treatment of viridans streptococcal endocarditis. Resistant organisms are most commonly seen in patients receiving penicillin prophylaxis for rheumatic heart disease. Treatment of endocarditis varies depending on whether the patient has native valves or prosthetic valves/material and whether the organism is penicillin susceptible. Refer to the American Heart Guidelines on Infective Endocarditis for a complete discussion and recommendations.
C. Other Viridans Streptococci–Related Infections
Viridans streptococci are normal flora of the gastrointestinal tract, respiratory tract, and the mouth. In many cases, isolation of viridans streptococci from a blood culture is considered to be a “contaminant” in the absence of signs or symptoms of endocarditis or other invasive disease. However, in children who are immunocompromised, have congenital or acquired valvular heart disease, or those who have indwelling lines, these viridans streptococci may be a cause of serious morbidity. About one-third of bacteremias in patients with malignancies may be due to bacteria from the Streptococcus viridans group. Mucositis and gastrointestinal toxicity from chemotherapy are among the risk factors for developing disease. Even in children with normal immune systems, viridans streptococci sometimes cause serious infections. For example, viridans streptococci isolated from an abdominal abscess after sustained rupture of the appendix represents a true pathogen. Streptococcus anginosus, a member of the Streptococcus viridans group, is seen as a cause of intracranial abscess (often as a complication of sinusitis) and abdominal abscesses. In patients with risk factors or signs/symptoms for subacute endocarditis, isolation of one of the members of the Streptococcus viridans group should prompt consideration and evaluation for possible endocarditis (see previous section).
Increasing prevalence of antibiotic resistance has been seen over the last 10 years in isolates of the streptococci viridans group. Penicillin resistance varies with geographic region, institution, and the populations tested, but has ranged from 30% to 70% in oncology patients. Cephalosporin resistance is also relatively common. Therefore, it is important to obtain antibiotic susceptibilities to the organism to select effective therapy. Vancomycin, linezolid, and quinupristin-dalfopristin are still effective against most isolates.
Baddour M: Infective endocarditis: diagnosis and management. Circulation 2005;111:3167 [PMID: 15956145].
Butler KM: Enterococcal infection in children. Semin Pediatr Infect Dis 2006;17:128 [PMID: 16934707].
PNEUMOCOCCAL INFECTIONS
 General Considerations
General Considerations
Sepsis, sinusitis, otitis media, pneumonitis, meningitis, osteomyelitis, cellulitis, arthritis, vaginitis, and peritonitis are all part of a spectrum of pneumococcal infection. Clinical findings that correlate with occult bacteremia in ambulatory patients include age (6–24 months), degree of temperature elevation (> 39.4°C), and leukocytosis (> 15,000/μL). Although each of these findings is in itself nonspecific, a combination of them should arouse suspicion. This constellation of findings in a child who has no focus of infection may be an indication for blood cultures and antibiotic therapy. The cause of most of such bacteremic episodes is pneumococci.
Streptococcus pneumoniae is a common cause of acute purulent otitis media and is the organism responsible for most cases of acute bacterial pneumonia in children. The disease is indistinguishable on clinical grounds from other bacterial pneumonias. Effusions are common, although frank empyema is less common. Abscesses also occasionally occur.
The incidence rate of pneumococcal meningitis has decreased since incorporation of the pneumococcal conjugate vaccine into the infant vaccine schedule. However, pneumococcal meningitis is still more common than Haemophilus influenzae type b meningitis. Pneumococcal meningitis, sometimes recurrent, may complicate serious head trauma, particularly if there is persistent leakage of CSF. This has led some physicians to recommend the prophylactic administration of penicillin or other antimicrobials in such cases.
Children with sickle cell disease, other hemoglobinopathies, congenital or acquired asplenia, and some immunoglobulin and complement deficiencies are unusually susceptible to pneumococcal sepsis and meningitis. They often have a catastrophic illness with shock and disseminated intravascular coagulation (DIC). Even with excellent supportive care, the mortality rate is 20%–50%. The spleen is important in the control of pneumococcal infection by clearing organisms from the blood and producing an opsonin that enhances phagocytosis. Autosplenectomy may explain why children with sickle cell disease are at increased risk of developing serious pneumococcal infections. Children with cochlear implants are at higher risk for pneumococcal meningitis.
S pneumoniae rarely causes serious disease in the neonate. Although S pneumoniae does not normally colonize the vagina, transient colonization does occur. Serious neonatal disease—including pneumonia, sepsis, and meningitis—may occur and clinically is similar to GBS infection.
Historically, penicillin was the agent of choice for pneumococcal infections, and some strains are still highly susceptible to penicillin. However, pneumococci with moderately increased resistance to penicillin are found in most communities. The prevalence of these relatively penicillin-resistant strains in North America varies geographically. Pneumococci with high-level resistance to penicillin and multiple other drugs are increasingly encountered throughout the United States. Pneumococci from normally sterile body fluids should be routinely tested for susceptibility to penicillin as well as other drugs.
Pneumococci have been classified into more than 90 serotypes based on capsular polysaccharide antigens. The frequency distribution of serotypes varies at different times, in different geographic areas, and with different sites of infection.
 Prevention
Prevention
Two pneumococcal vaccines are licensed for use in children in the United States: 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. The 13-valent pneumococcal conjugate vaccine was licensed in 2010 (replacing the 7-valent pneumococcal vaccine). This vaccine contains antigens from 13 pneumococcal serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 10F, and 23F), and is the vaccine currently recommended for routine use in the infant and childhood immunization schedule. Use of the 13-valent conjugate vaccine is important in the prevention of pneumococcal disease because young children (age < 2 years), who are most at risk for the disease, are unable to immunologically mount a predictable response to the 23-valent polysaccharide vaccine. The 13-valent vaccine is currently recommended for (1) infant primary series and childhood booster dosing (replaces the 7-valent vaccine), (2) as a single supplemental dose in healthy children 14– 59 months of age who were fully immunized with the 7-valent pneumococcal conjugate vaccine, and (3) use in children aged less than 18 years who have certain underlying medical conditions that put them at high risk for pneumococcal disease. This vaccine and indications for use is discussed in detail in Chapter 10. For children > 2 years of age who are at high risk for invasive pneumococcal disease (sickle cell anemia, anatomic or functional asplenia, HIV-infected children, and persons with certain chronic illnesses), the 23-valent pneumococcal vaccine is recommended 8 weeks following completion of the pneumococcal conjugate vaccine series (see Chapter 10). A second dose of the 23-valent pneumococcal vaccine should be given 5 years after the first dose in children with HIV infection, sickle cell disease, functional or anatomic asplenia, or other immunocompromising conditions.
When a cochlear implant, splenectomy, or immune-compromising therapy is anticipated, the child should complete immunization with the 13-valent pneumococcal conjugate vaccine at least 2 weeks prior to surgery or institution of immune-compromising therapy if possible. If this is not possible due to the urgency of the procedure or therapy, the child should receive immunization as soon as possible thereafter. In children greater than 2 years of age, the 23-valent pneumococcal polysaccharide vaccine can be given at least 8 weeks after completion of the 13-valent pneumococcal conjugate vaccine (see Chapter 10).
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
In pneumococcal sepsis, fever usually appears abruptly, often accompanied by chills. There may be no respiratory symptoms. In pneumococcal sinusitis, mucopurulent nasal discharge may occur. In infants and young children with pneumonia, fever, and tachypnea without auscultatory changes are the usual presenting signs. Respiratory distress is manifested by nasal flaring, chest retractions, and tachypnea. Abdominal pain is common. In older children, the adult form of pneumococcal pneumonia with signs of lobar consolidation may occur, but sputum is rarely bloody. Thoracic pain (from pleural involvement) is sometimes present, but is less common in children. With involvement of the right hemidiaphragm, pain may be referred to the right lower quadrant, suggesting appendicitis. Vomiting is common at onset but seldom persists. Convulsions are relatively common at onset in infants.
Meningitis is characterized by fever, irritability, convulsions, and neck stiffness. The most important sign in very young infants is a tense, bulging anterior fontanelle. In older children, fever, chills, headache, and vomiting are common symptoms. Classic signs are nuchal rigidity associated with positive Brudzinski and Kernig signs. With progression of untreated disease, the child may develop opisthotonos, stupor, and coma.
B. Laboratory Findings
Leukocytosis is often pronounced (20,000–45,000/μL), with 80%–90% polymorphonuclear neutrophils. Neutropenia may be seen early in very serious infections. The presence of pneumococci in the nasopharynx is not a helpful finding, because up to 40% of normal children carry pneumococci in the upper respiratory tract. Large numbers of organisms are seen on Gram-stained smears of endotracheal aspirates from patients with pneumonia. In meningitis, CSF usually shows an elevated white blood cell (WBC) count of several thousand, chiefly polymorphonuclear neutrophils, with decreased glucose and elevated protein levels. Gram-positive diplococci may be seen on some (but not all) stained smears of CSF sediment. Antigen detection tests are not useful. Isolation of S pneumoniae from a normally sterile site (eg, blood, cerebrospinal joint fluid, middle ear fluid) or from a suppurative focus confirms the diagnosis.
 Differential Diagnosis
Differential Diagnosis
There are many causes of high fever and leukocytosis in young infants; 90% of children presenting with these features have a disease other than pneumococcal bacteremia, such as human herpesvirus 6, enterovirus, or other viral infection; urinary tract infection; unrecognized focal infection elsewhere in the body; or early acute shigellosis.
Infants with upper respiratory tract infection who subsequently develop signs of lower respiratory disease are most likely to be infected with a respiratory virus. Hoarseness or wheezing is often present. A radiograph of the chest typically shows perihilar infiltrates and increased bronchovascular markings. Viral respiratory infection often precedes pneumococcal pneumonia; therefore, the clinical picture may be mixed.
Staphylococcal pneumonia may be indistinguishable early in its course from pneumococcal pneumonia. Later, pulmonary cavitation and empyema occur.
In primary pulmonary tuberculosis, children do not have a toxic appearance, and radiographs show a primary focus associated with hilar adenopathy and often with pleural involvement. Miliary tuberculosis presents a classic radiographic appearance.
Pneumonia caused by Mycoplasma pneumoniae is most common in children aged 5 years and older. Onset is insidious, with infrequent chills, low-grade fever, prominent headache and malaise, cough, and, often, striking radiographic changes. Marked leukocytosis (> 18,000/μL) is unusual.
Pneumococcal meningitis is diagnosed by lumbar puncture. Without a Gram-stained smear and culture of CSF, it is not distinguishable from other types of acute bacterial meningitis.
 Complications
Complications
Complications of sepsis include meningitis and osteomyelitis; complications of pneumonia include empyema, parapneumonic effusion, and, rarely, lung abscess. Mastoiditis, subdural empyema, and brain abscess may follow untreated pneumococcal otitis media. Both pneumococcal meningitis and peritonitis are more likely to occur independently without coexisting pneumonia. Shock, DIC, and Waterhouse-Friderichsen syndrome resembling meningococcemia are occasionally seen in pneumococcal sepsis, particularly in asplenic patients. Hemolytic-uremic syndrome may occur as a complication of pneumococcal pneumonia or sepsis.
 Treatment
Treatment
A. Specific Measures
All S pneumoniae isolated from normally sterile sites should be tested for antimicrobial susceptibility. The term “nonsusceptible” is used to describe both intermediate and resistant isolates. Strains that are nonsusceptible to penicillin, ceftriaxone (or cefotaxime), and other antimicrobials are increasingly common globally. Antimicrobial susceptibility breakpoints for S pneumoniae to penicillin and ceftriaxone/cefotaxime are based on whether the patient has meningitis and the drug route (oral vs intravenous), see Table 42–2. Therapy of meningitis, empyema, osteomyelitis, and endocarditis due to nonsusceptible S pneumoniae is challenging, because penetration of antimicrobials to these sites is limited. Infectious disease consultation is recommended for advice regarding these problems. For empiric therapy of serious or life-threatening infections pending susceptibility test results, vancomycin and ceftriaxone (or cefotaxime) are recommended.
Table 42–2. Penicillin breakpoints (minimum inhibitory concentrations [MIC]) for Streptococcus pneumoniae by susceptibility category—Clinical and Laboratory Standards Institute, 2008.
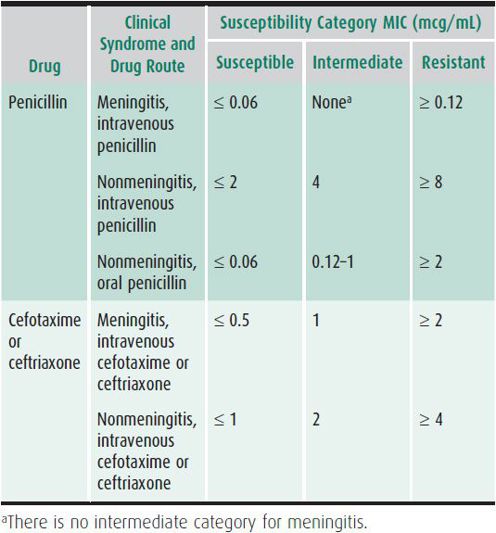
1. Bacteremia—In studies done prior to immunization of young children with conjugated pneumococcal vaccine, 3%–5% of blood cultures in patients younger than 2 years of age yielded S pneumoniae. These percentages decreased with the addition of conjugated pneumococcal vaccine to the vaccine schedule. The current 13-valent pneumococcal vaccine contains antigens to the pneumococcal serotypes that cause about 65% of invasive pneumococcal disease. Many experts treat suspected bacteremia in children that are not seriously ill with ceftriaxone (50 mg/kg, given intramuscularly or intravenously). Compared with oral amoxicillin (80–90 mg/kg/d), ceftriaxone may reduce fever and the need for hospitalization. However, meningitis occurs with the same frequency despite presumptive therapy. All children with blood cultures that grow pneumococci should be reexamined as soon as possible. The child who has a focal infection, such as meningitis, or who appears septic should be admitted to the hospital to receive parenteral antimicrobials. If the child is afebrile and appears well or mildly ill, outpatient management is appropriate. Severely ill or immunocompromised children, in whom invasive infection with S pneumoniae is suspected, should be treated with vancomycin (in addition to other appropriate antibiotics to cover other suspected pathogens). If meningitis is also suspected, use ceftriaxone or cefotaxime in addition to vancomycin until the susceptibilities of the organism are known.
2. Pneumonia—For infants (1 month of age or older) with susceptible organisms appropriate regimens include ampicillin (150–200 mg/kg/d intravenously in four divided doses) aqueous penicillin G (250,000–400,000 U/kg/d, given intravenously in four to six divided doses), cefotaxime (50 mg/kg intravenously every 8 hours), or ceftriaxone (50 mg/kg intravenously every 12–24 hours). If susceptibilities are not known and the patient is severely ill or immunocompromised, vancomycin should be used as part of the regimen to provide coverage for penicillin- or cephalosporin-resistant pneumococcus. Once results of susceptibility testing are available, the regimen can be tailored. Mild pneumonia may be treated with amoxicillin (80–90 mg/kg/d) for 7–10 days. Alternative regimens include oral macrolides (resistance may be high) and cephalosporins.
3. Otitis media—Most experts recommend oral amoxicillin (80–90 mg/kg/d, divided in two doses) as first-line therapy. The standard course of therapy is 10 days; however, many physicians treat uncomplicated, mild cases in children 6 years of age or older for 5–7 days. Treatment failures may be treated with amoxicillin-clavulanate (80–90 mg/kg/d of the amoxicillin component in the 14:1 formulation), intramuscular ceftriaxone, cefuroxime axetil, or cefdinir. Azithromycin can also be used in patients with type I hypersensitivity reactions to penicillin or cephalosporin, but resistance to macrolides may be high.
4. Meningitis—Until bacteriologic confirmation and susceptibility testing are completed, patients should receive vancomycin (60 mg/kg/d, given intravenously in four divided doses) and cefotaxime (225–300 mg/kg/d intravenously in four divided doses), OR vancomycin (see previous dosage) and ceftriaxone (100 mg/kg/d, given intravenously in two divided doses). Patients with serious hypersensitivity to beta-lactam antibiotics allergy (eg, penicillins, cephalosporins) can be treated with a combination of vancomycin (see previous dosage) and rifampin (20 mg/kg/day in two divided doses). Use of vancomycin alone or use of rifampin alone is not recommended. Vancomycin and meropenem is an alternative for penicillin or cephalosporin allergic patients and this regimen provides additional gram-negative coverage until culture and susceptibility results are obtained. Corticosteroids (dexamethasone, 0.6 mg/kg/d, in four divided doses for 4 days) are controversial but are recommended by many experts as adjunctive therapy for pneumococcal meningitis. A repeat lumbar puncture at 24–48 hours should be considered to ensure sterility of the CSF if dexamethasone is given, if resistant pneumococci are isolated, or if the patient is not demonstrating expected improvement after 24–48 hours on therapy.
If the isolate is determined to be penicillin-susceptible, aqueous penicillin G can be administered (300,000– 400,000 U/kg/d, given intravenously in four to six divided doses for 10–14 days). Alternatively, use of ceftriaxone or cefotaxime is an acceptable alternative therapy for penicillin- and cephalosporin-susceptible isolates. Consult an infectious disease specialist or the Red Book (American Academy of Pediatrics, 2012) for a complete discussion of pneumococcal meningitis and for therapeutic options for isolates that are nonsusceptible to penicillin or cephalosporins.
 Prognosis
Prognosis
In children, case fatality rates of less than 1% should be achieved except for meningitis, where rates of 5%–20% still prevail. The presence of large numbers of organisms without a prominent CSF inflammatory response or meningitis due to a penicillin-resistant strain indicates a poor prognosis. Serious neurologic sequelae, particularly hearing loss, are frequent following pneumococcal meningitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

































