Infant of the Diabetic Mother
Joseph B. Warshaw
William W. Hay Jr.
INTRODUCTION AND DEFINITIONS
Diabetes mellitus is the most common medical complication of pregnancy. Diabetes occurs in two forms during pregnancy, overt and gestational. Approximately 1 in 200 pregnancies is complicated by overt diabetes. Overt diabetes refers to clinically apparent diabetes diagnosed prior to or very early in pregnancy. Gestational diabetes develops in an additional 2% to 3% of pregnancies, accounting for 90% of all pregnancies complicated by diabetes of any kind. Gestational diabetes refers to patients in whom diabetes, defined as glucose intolerance of variable severity, develops during later pregnancy and disappears after delivery. Despite advances in perinatal care since the 1970s, diabetes in pregnancy remains a significant cause
of perinatal morbidity and mortality. Diabetes in pregnancy is classified according to the degree of glycemia (Table 63.1). The term class A diabetic often is used interchangeably with gestational diabetic or chemical diabetic (class A1, fasting blood glucose less than 105 mg/dL), but this use is not totally accurate because women with gestational diabetes with a fasting hyperglycemia of greater than 105 mg/dL may require insulin and are placed into class A2. This classification has been modified slightly over many years, primarily by noting whether the mother is obese, noting whether she simply has impaired gestational glucose tolerance without baseline changes in glycemia, and including other subgroups of women with more chronic complications of diabetes such as kidney disease, advanced stages of retinopathy, and pancreatic disease.
of perinatal morbidity and mortality. Diabetes in pregnancy is classified according to the degree of glycemia (Table 63.1). The term class A diabetic often is used interchangeably with gestational diabetic or chemical diabetic (class A1, fasting blood glucose less than 105 mg/dL), but this use is not totally accurate because women with gestational diabetes with a fasting hyperglycemia of greater than 105 mg/dL may require insulin and are placed into class A2. This classification has been modified slightly over many years, primarily by noting whether the mother is obese, noting whether she simply has impaired gestational glucose tolerance without baseline changes in glycemia, and including other subgroups of women with more chronic complications of diabetes such as kidney disease, advanced stages of retinopathy, and pancreatic disease.
TABLE 63.1. CLASSIFICATION OF DIABETES COMPLICATING PREGNANCY | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FETAL GLUCOSE SUPPLY AND METABOLISM
Glucose is transported across the placenta from maternal to fetal plasma according to the maternal–fetal arterial plasma glucose concentration gradient. The transport of glucose across the placenta, as with all membranes, follows saturation-limited or Michaelis-Menten kinetics. The Km for transport is in the high range of normal maternal plasma glucose concentrations, allowing direct control of fetal glucose concentration by that of the mother. Glucose transport across the placenta is facilitated by specific transporter molecules; GLUT 1, a low-affinity transporter, which is the predominant isoform in the placenta; and GLUT 3, a high-affinity transporter that perhaps allows for enhanced transport even with maternal hypoglycemia. As a result of these transport mechanisms in the placenta, fetal plasma glucose concentrations are directly related to, but are about 20% less than those of the mother.
High concentrations of fetal glucose in response to maternal hyperglycemia stimulate the fetal pancreatic islet to secrete insulin. Fetal islet cell volume has been shown to be proportional to maternal glucose concentrations, and fetal macrosomia has been associated with increased plasma insulin and C peptide concentrations in cord blood. Insulin functions as a fetal growth hormone, resulting in the characteristic macrosomia of infants of diabetic mothers (IDMs). This macrosomia is primarily due to increased subcutaneous white fat deposition, the product of excessive glucose, glycerol derived from glucose, and fatty acids and triglycerides from the maternal plasma that in the presence of insulin are synthesized into fat stores in fetal adipocytes. In utero, IDMs are not overgrown until sometime after week 26 or 27; this staging may be related to the development of insulin receptors. Evidence also indicates that insulinlike growth factor–I (IGF-I) contributes to fetal macrosomia. IGF-I may affect muscle mass more than insulin does, although both insulin and IGF-I act to promote cellular hypertrophy and hyperplasia. Insulin also decreases protein breakdown.
The risk of fetal macrosomia is increased when the mean maternal glucose concentration exceeds 130 mg/dL. Infants are the largest when maternal glucose concentrations are episodically increased, particularly following a meal (meal-associated or pulsatile hyperglycemia), as this form of hyperglycemia, when translated into surges of hyperglycemia in the fetus, has the greatest effect on fetal pancreatic insulin secretion.
Measurement of glycosylated hemoglobin (HbA1c) provides an important index of longer-term diabetes control. Generally, normal levels of HbA1c are less than 8%. Many studies have demonstrated that elevated HbA1c concentrations are associated with increased perinatal morbidity. The obstetric goal should be maintenance of fasting glucose concentrations at less than 100 mg/dL and other plasma glucose concentrations at less than 130 mg/dL. Triglyceride and free fatty acid concentrations also should be monitored and kept in the normal range, as fetal adiposity is directly related to the maternal plasma concentrations of these substrates as well as glucose. Maternal obesity and subsequent pregnancy weight gain are more important risk factors for fetal macrosomia than either maternal glucose concentration or free fatty acid and triglyceride concentrations alone. All three indices should be monitored frequently in the pregnant woman with diabetes of any kind.
GENERAL CHARACTERISTICS OF INFANTS OF DIABETIC MOTHERS
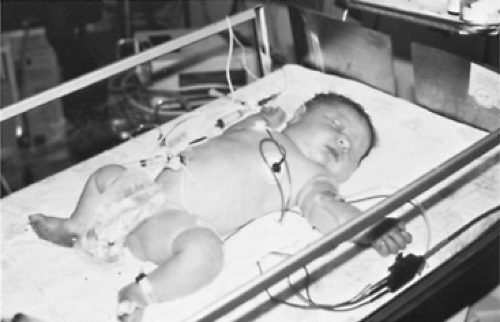
IDMs have a characteristic appearance, with macrosomia (total body obesity), abundant adipose tissue, and a cherubic facial appearance; their head circumference, however, is similar to that of age-matched normal infants, because insulin does not influence brain growth. A typical IDM is shown in Fig. 63.1. Adipose tissue of IDMs often exceeds the normal 12% to 18% of body weight. The IDM also has an increased glycogen
content in the liver, kidney, skeletal muscle, and heart. The growth hormone-like effects of insulin result in increased linear growth.
content in the liver, kidney, skeletal muscle, and heart. The growth hormone-like effects of insulin result in increased linear growth.
Macrosomia contributes to birth trauma and birth asphyxia. Birth trauma can and should be prevented by frequent ultrasounds of the fetus documenting the degree of macrosomia, pelvic outlet size determinations, and cesarean section delivery. Perinatal asphyxia may occur in 25% of infants of insulin-dependent diabetic mothers. It is more common in macrosomic infants in part because of their size and in part because of the associated metabolic abnormalities. Asphyxia correlates with maternal hyperglycemia before delivery, particularly during labor. Intrapartum maternal and fetal hyperglycemia may result in increased fetal oxygen requirements. This places the fetus at risk of hypoxemia and acidosis during delivery when uterine contractions normally reduce uterine blood flow.
Asphyxia is also more common with maternal nephropathy. Nephropathy is associated with decreased placental blood flow; this association is confounded by the association of nephropathy with more severe maternal hyperglycemia, itself inversely related to placental blood flow.
Finally, changes in hemoglobin in severe diabetes may interfere with oxygen transport. Glycosylation of hemoglobin as with HbA1C increases the affinity of hemoglobin for oxygen, thereby decreasing the uterine venous PO2 and transfer of oxygen to the fetus. Furthermore, decreases in 2,3-diphosphoglycerate (2,3-DPG) seen with recurrent ketoacidosis can add to the decrease in uterine venous PO2. Further potential reduction in oxygen transfer to the fetus may be caused by increased diffusion distance in the thickened basement membranes or edematous trophoblastic villi in diabetic placentas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree