Fig. 7.1
TVS showing uniformly echogenic endometrium in the secretory phase
Normal Sonographic Appearance of Endometrium in Postmenopausal Women
Postmenopausal endometrium is thin, homogenous, and echogenic (Fig. 7.2). In 2001, the Society of Radiologists in an ultrasound consensus panel recommended a cutoff value of 5 mm endometrial thickness in postmenopausal women with bleeding for intervention which conferred 96 % sensitivity for the detection of endometrial cancer [3]. ACOG guidelines recommend that an endometrial thickness up to 4 mm reliably excludes endometrial cancer [4].
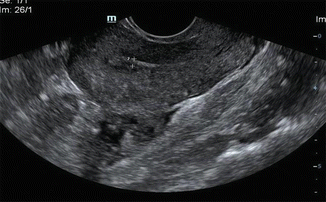

Fig. 7.2
TVS showing postmenopausal thin endometrium
Endometrial Measurement on Transvaginal Sonography
The endometrium is best measured in midline sagittal scan of the uterus at the thickest point and includes both layers of echogenic endometrium (Fig. 7.3). Small amount of fluid within the endometrial cavity may be a normal finding. This fluid should be excluded while measuring the endometrium (Fig. 7.4).
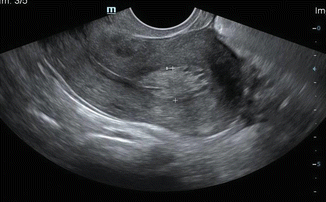
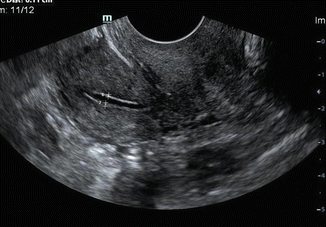

Fig. 7.3
TVS showing an endometrial thickening of 1.1 cm (note the correct placement of calipers)

Fig. 7.4
TVS showing accurate placement of calipers to measure endometrial thickness, if there is fluid within the endometrium
Transvaginal Sonography in Premenopausal Bleeding
Ten to twenty percent of endometrial cancers are diagnosed in premenopausal women with abnormal uterine bleeding [5].
Transvaginal ultrasonography is the initial screening modality in premenopausal bleeding to assess the endometrial thickness and to rule out structural abnormalities like endometrial polyps, submucosal fibroids, polycystic ovaries, or other hormone-producing tumors of the ovary.
Sonosalpingography and other invasive tests like hysteroscopy and endometrial biopsy are important in this age group to rule out endometrial malignancy if bleeding persists despite medical management.
Transvaginal Sonography in Postmenopausal Bleeding
Ten percent of cases with postmenopausal bleeding have endometrial cancer [3]. Any vaginal bleeding in a postmenopausal woman should be investigated to exclude malignancy, the other causes being polyps, endometrial atrophy, atrophic vaginitis, endometrial hyperplasia, hormone therapy, medications, and other cancers.
The ultrasound appearances in endometrial malignancy in a postmenopausal woman with bleeding are as given below (Figs. 7.5, 7.6, and 7.7):
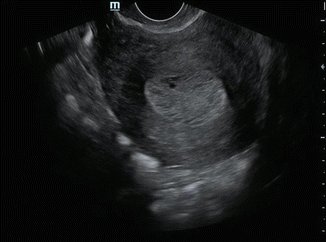
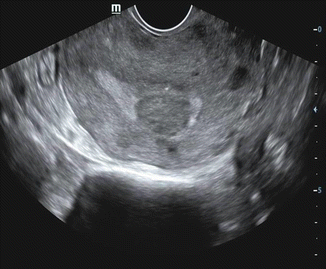
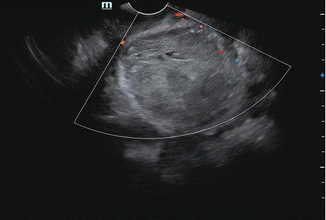

Fig. 7.5
TVS showing 20 mm thickened endometrium with cystic areas

Fig. 7.6
TVS showing hypoechoic polypoidal mass within the endometrium

Fig. 7.7
TVS with Doppler showing diffuse infiltrating endometrial cancer with uterine enlargement
1.
Uniformly thickened echogenic endometrium. Cystic changes within the thickened endometrium may be seen in 5–15 % case of endometrial cancer.
2.
Focal endometrial thickening due to polypoidal lesions.
3.
Heterogenous mass replacing the endometrium.
4.
Diffuse uterine enlargement due to large tumors infiltrating the myometrium.
5.
Tumors in the lower endometrium may obstruct the endometrial cavity, resulting in hematometra.
Role of Transvaginal Sonography in Staging of Endometrial Cancers
Once the diagnosis is established by biopsy, transvaginal ultrasound can help in staging the tumor to a certain extent, by determining the involvement of the myometrium and depth of myometrial invasion. Myometrial invasion is seen as irregularity of the endometrial–myometrial border with disruption of subendometrial echo. The tumor–myometrial interface would be preserved in early lesions and poorly defined with irregular margins in advanced disease. However, this differentiation is often difficult. It is stated that ultrasound has accuracies ranging from 73 % to 84 % in assessing myometrial invasion [6]. 3D ultrasonography with virtual navigation software may help in better identifying myometrial invasion in the future [7].
The Endometrium and Hormone Therapy
For women receiving hormone therapy, endometrial thickening varies with the regimen used—continuous or cyclical.
Women taking sequential continuous estrogen–progestin replacement regimen in perimenopausal age group have endometrial thickening, almost 15 mm during estrogen phase and then decreasing after progesterone is added. The endometrial thickness in such cases should be measured immediately after the progesterone phase when it is the thinnest. The cutoff threshold is 5 mm.
Approximately 50 % of postmenopausal women taking unopposed continuous estrogen alone may have an endometrium exceeding 8 mm in diameter (range 6–10 mm). A thickness of 8 mm is considered the upper limit of normal if the patient is asymptomatic. However, if the woman reports postmenopausal bleeding, a thickness cutoff of 5 mm is used for deciding upon further investigations as in the other postmenopausal women [8].
The Endometrium and Tamoxifen
The use of tamoxifen as an adjunctive treatment for breast cancer has resulted in an increased prevalence of endometrial hyperplasia with or without cystic changes. Subendometrial cystic atrophy is a benign process that can occur as part of tamoxifen-associated endometrial changes.
Most women with thickened endometrium are asymptomatic. Some investigators have proposed a cutoff of 5–8 mm for diagnosing endometrial abnormalities in asymptomatic postmenopausal women receiving tamoxifen [8].
Transvaginal sonography has a sensitivity of 63.3 % and specificity of only 60.4 % for the detection of endometrial malignancy in patients on tamoxifen even when the cutoff is 9 mm.[9]. Hence, routine ultrasound surveillance in asymptomatic women using tamoxifen is of limited use.
Advantages of Ultrasonography
1.
Transvaginal sonography in a postmenopausal woman with bleeding has a high negative predictive value and hence is a good screening modality.
2.
Transvaginal sonography avoids endometrial biopsy in majority of postmenopausal bleeding with normal/atrophic endometrium [10].
3.
Transvaginal sonography (TVS) identifies appropriate cases with endometrial thickening in women with abnormal uterine bleeding for further investigations.
4.
TVS can classify endometrial lesions as focal (polyps, submucosal fibroids) and diffuse (endometrial hyperplasia). Diffuse lesions may require biopsy for further delineation, whereas focal lesions may require more invasive work-up with hysteroscopy and targeted biopsy [8] or removal.
Pitfalls in Diagnosis and Staging Using TVS
1.
It is not possible to get a reliable measurement of endometrial thickness by TVS in all cases. Preexisting conditions like fibroid, adenomyosis, and previous uterine surgery make the accurate assessment of endometrial thickening and myometrial invasion difficult. In such cases, alternative assessment should be suggested.
2.
If there is uterine enlargement, the entire endometrium up to the fundal region may not be optimally visualized by TVS probe. Maneuvers like anterior and extreme probe angulation and abdominal compression may bring the uterus to the focal zone of the transducer.
3.
In some cases, the sonographic appearance of a diffusely thick endometrium may be due to focal isoechoic endometrial polyps.
4.
Bulky endocavitary polypoid tumor that produces myometrial thinning may be confused with advanced and invasive tumors on staging.
5.
It is difficult to delineate cervical and parametrial infiltration even with high-resolution vaginal probe.
Role of Doppler
The role of Doppler is limited as there is significant overlap in Doppler indices in benign and malignant conditions. Color and power Doppler reveals diffuse or focal increased vascularity in endometrial carcinoma, and spectral Doppler may show low-resistance flow pattern. Color and power Doppler may help in ensuring that biopsies are targeted from areas of increased blood flow (Fig. 7.8).
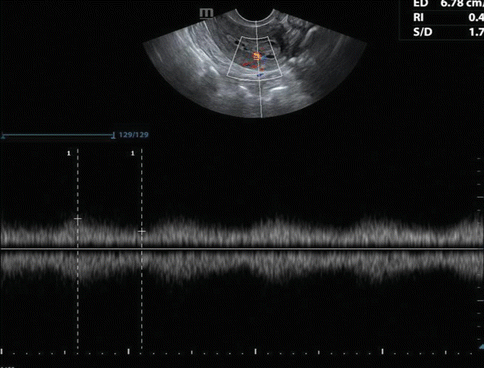

Fig. 7.8
TVS with Doppler showing low-resistance flow pattern in endometrial malignancy
Role of Saline Infusion Sonohysterography (Sonosalpingography)
Hysterosonography, i.e., transvaginal US evaluation of the uterus after intracavitary saline infusion, has been used for evaluating myometrial invasion, with accuracies ranging from 84 % to 89 % [10]. It may help to reach a conclusive diagnosis in difficult situations (Figs. 7.9 and 7.10).
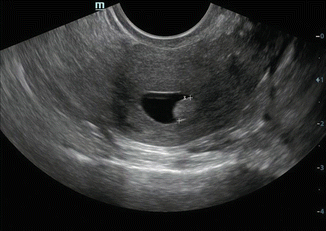
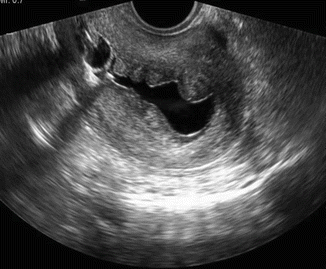

Fig. 7.9
Sonosalpingography showing small uniformly hyperechoic endometrial polyp

Fig. 7.10
Sonosalpingography showing polypoidal endometrial thickening
Advantages of Sonosalpingography over TVS
When fluid outlines the endometrium, the endometrium can be better assessed for irregularities and polypoid masses that are missed at conventional transvaginal US. It can thus differentiate focal from diffuse endometrial thickening better than TVS.
It helps to differentiate between polyps and submucosal fibroids. A polyp is seen arising from the endometrium, while normal endometrium is seen overlying submucous fibroid.
Sonohysterography plays an important role as an adjuvant to endovaginal sonography in patients on tamoxifen by delineating endometrial and subendometrial disorders and selecting patients requiring hysteroscopy versus sampling in symptomatic women with vaginal bleeding.
The role of sonosalpingography is controversial in endometrial malignancy as this procedure may disseminate malignant cells into the peritoneal cavity [10].
Sonosalpingography Versus Hysteroscopy
Sonohysterography is less invasive, less expensive, well tolerated, and without major complications compared to hysteroscopy.
Sonohysterography and hysteroscopy show similar performance characteristics with regard to sensitivity (95–96 %) and specificity (88–90 %) for detecting and characterizing focal endometrial abnormalities [8]. However, hysteroscopy enables sampling and helps in tissue diagnosis.
Role of CT Scan
CT remains the imaging modality used most frequently in clinical practice for preoperative evaluation as it is widely available. In advanced disease where surgery is not a treatment option, CT can be used for preoperative staging and follow-up.
Endometrial cancer is isodense to myometrium in plain CT scan and shows relatively low attenuation compared with myometrium after intravenous contrast. The sensitivity, specificity, and accuracy of 16-slice multidetector CT scan in evaluating myometrial invasion were 100 %, 80 %, and 95 %, respectively, and for assessing cervical infiltration were 78 %, 83 %, and 81 %, respectively [11]. The performance of higher slice CT may be marginally better.
CT scan cannot differentiate between stage 1a, b, and II disease and also cannot detect early cervical, vaginal, and parametrial involvement. However, CT can demonstrate obvious parametrial involvement, infiltration of the bladder and rectum, pelvic and para-aortic nodal involvement, and peritoneal metastasis in advanced disease. Peritoneal metastasis on CT appears as peritoneal thickening with soft tissue deposits and ascites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


