Blood supply to, and venous drainage from, the hypothalamo–pituitary axis
Histology of the hypothalamo-pituitary region
There are three types of hypothalamic neurosecretory cells. Magnocellular neurons secrete arginine vasopressin (AVP) or oxytocin (OXY). The cell bodies, which are located in the supraoptic or paraventricular hypothalamic nucleus, project their neuronal processes via the pituitary stalk into the posteriorneural lobe and neurohormones are released from nerve endings into the blood. Hypophysiotrophic neurons are located in nuclear groups, including the paraventricular and arcuate and the arcuate nucleus in the medial basal hypothalamus. The hypophysiotrophic neuropeptides are released into the hypothalamo–hypophysial portal system and regulate the secretion of hormones from the pituitary. Hypophysiotrophic neuropeptides include thyrotrophin-releasing hormone (TRH), corticotrophin-releasing hormone (CRH), somatostatin, growth-hormone-releasing hormone (GHRH), gonadotrophin-releasing hormone (GnRH) and dopamine.
Projection neurons are found in the paraventricular and arcuate nuclei and in the lateral hypothalamic area. These neurons project to autonomic preganglionic neurons in the brain stem and spinal cord.
Microscopic examination of the conventionally stained adenohypophysis reveals three distinctive cell types termed acidophils, basophils and chromophobes. This pattern of staining reflects the chemical character of intracellular hormone-laden granules within the pituitary cells.
The neurohypophysis is an extension of the hypothalamus and is composed of bundles of axons from hypothalamic neurosecretory neurons intermixed with glial cells.
Embryology of the hypothalamo–pituitary region
The anterior lobe of the pituitary gland or adenohypophysis develops from an evagination of ectodermal cells of the oropharynx in the primitive gut and is recognisable at weeks 4–5 of gestation. This evagination, known as the Rathke pouch, is eventually pinched off from the oral cavity and becomes separated by the sphenoid bone of the skull. The lumen of the pouch is reduced to a small cleft, while the upper portion of the pouch surrounds the neural stalk and forms the pars tuberalis. This, together with the anterior lobe, is called the adenohypophysis. When cells from the Rathke pouch are left behind and form tumours, these are called craniopharyngiomas.
The posterior lobe develops from neural crest cells as a downward evagination of the floor of the third ventricle of the brain. The lumen of this pouch closes as the sides fuse to form the neural stalk, while the upper portion of the pouch forms a recess in the floor of the third ventricle known as the median eminence.
The neural stalk together with the median eminence forms the infundibular stalk and this, together with the posterior lobe, is collectively termed the neurohypophysis. The cleft-like remnant of the Rathke pouch demarcates the boundary between the anterior and posterior lobes of the pituitary gland. The hypothalamo–pituitary axis is established by week 20 of gestation. The development of different types of hormone-secreting cells in adenohypophysis involves sequential expression of transcription factors coded by number of genes.
Physiology of the hypothalamic and pituitary glands
Functions of the hypothalamus include the control of the autonomic nervous system and the regulation of body temperature, hunger and thirst; it also has a role in memory, behaviour and emotional responses. In this chapter, we restrict discussion to the regulation of the endocrine system.
Anterior Pituitary Gland
The anterior pituitary gland is regulated by three interacting elements: hypothalamic input (releasing and inhibitory factors), feedback effects of circulating hormones and paracrine and autocrine secretions of the pituitary gland itself. From a clinical perspective, the anterior pituitary gland synthesizes and secretes six major hormones: growth hormone (GH, also called somatotrophin), thyroid-stimulating hormone (TSH, also called thyrotrophin), adrenocorticotrophin (ACTH), follicle-stimulating hormone (FSH), luteinising hormone (LH) and prolactin (PRL).
The physiological control of TSH and ACTH is described in Chapter 17.
Growth hormone
GH is synthesised and secreted by cells called somatotrophs in the anterior pituitary gland. Somatotrophs constitute 40–50% of the total cells in the anterior pituitary gland. GH is a single-chain polypeptide containing 191 amino acids and shares structural homologies with PRL and human chorionic gonadotrophin (hCG), the latter being a GH variant synthesised exclusively in the placenta. GH predominantly affects growth, metabolism, cell differentiation and lactation. The effects of GH on protein metabolism include increases in amino acid uptake and protein synthesis and a decrease in the oxidation of proteins. GH enhances the metabolism of fats by stimulating triglyceride breakdown and oxidation in adipocytes. In carbohydrate metabolism, GH is one of the hormones that maintain blood glucose levels within the normal range. It suppresses insulin-mediated glucose uptake in peripheral tissues and insulin-mediated glucose synthesis in the liver. Somewhat paradoxically, GH stimulates insulin secretion and GH administration can lead to hyperinsulinaemia.
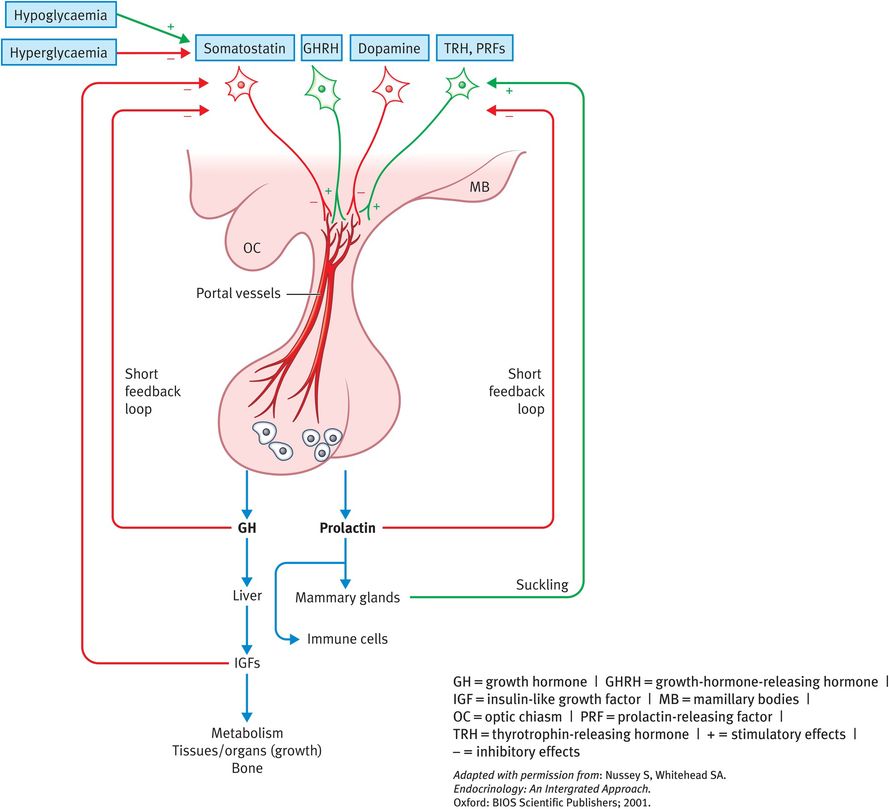
GH exerts its effects directly and indirectly through the family of insulin-like growth factors (IGFs). The IGF family consists of three members (insulin, IGF-I and IGF-II) that share common structural similarities. Insulin, IGF-I and IGF-II have metabolic functions, but they also have important roles in cellular proliferation and the functions of differentiated tissues (mediated by the IGF-I receptor). IGF-II is a major fetal growth factor. IGF-I is more important for postnatal growth. Insulin also plays a role in fetal growth. A reflection of this role is the development of fetal macrosomia in pregnancies associated with maternal diabetes. Synthesis and secretion of GH is stimulated by GH-releasing hormone (GHRH) and inhibited by somatostatin, both of which are produced by the hypothalamus (Figure 16.2). GHRH is released from nerve terminals in the median eminence and transported to the anterior pituitary gland via the hypophyseal portal capillaries. Somatostatin is a 14-amino-acid peptide that is synthesised in hypothalamic neurons mainly located in the anterior periventricular nuclei. Factors affecting GHRH and somatostatin secretion include sleep, exercise, stress and blood glucose levels. In addition, IGF-I provides negative feedback to the pituitary glands and hypothalamus. Finally, estradiol acts to increase the sensitivity of tissues to GH.
Actions and regulation of growth hormone and PRL
GH production begins early in fetal life and continues throughout life, although at a progressively lower rate. Mean daily GH secretion is pulsatile. It is high in childhood with a peak at puberty and secretion falls during adulthood. In the adult human, approximately five pulses of GH are secreted during a 24-hour period with a larger peak occurring at the onset of sleep at night. The half-life of GH in the circulation is about 20 minutes.
Prolactin
PRL is produced and secreted by the lactotrophs in the anterior pituitary gland. Lactotrophs constitute 10–15% of the anterior pituitary cells. PRL is a single-chain protein made up of 199 amino acids with three disulphide bridges. It shares strong structural homologies with GH and the cell surface receptors of PRL and GH are also similar. Estrogen stimulates the proliferation of pituitary lactotroph cells, resulting in an increased quantity of these cells in premenopausal women, especially during pregnancy.
In animals, PRL has a plethora of actions but in humans it is mainly involved in preparation of the female breast for lactation. PRL also binds to specific receptors in the gonads, lymphoid cells and liver. Other roles are poorly understood, although there is widespread tissue expression of PRL receptors. It is also notable that men have the same circulating concentrations of PRL as nonlactating women. PRL has effects on the hypothalamo–pituitary–gonadal axis, it can inhibit pulsatile secretion of GnRH from the hypothalamus and it can alter the activity of certain steroidogenic enzymes.
PRL secretion is regulated by a dual hypothalamic inhibitory and stimulatory system. In addition, it can regulate its own secretion through a short feedback loop.
PRL secretion is pulsatile and increases with sleep. The control of PRL release by the hypothalamus is unique in that the predominant hypothalamic influence is inhibitory, whereas it is stimulatory for all other hormones. Thus, damage to the hypothalamic control causes increased PRL levels. Dopamine, released into the hypophysial portal veins from nerve terminals in hypothalamus is the main neurohormone inhibiting PRL secretion. Somatostatin also has an inhibitory effect. Stimulatory factors include TRH, other releasing factors, pregnancy, lactation (suckling), estrogen, opioids, dopamine D2 receptor antagonists, sleep and stress.
Gonadotrophins
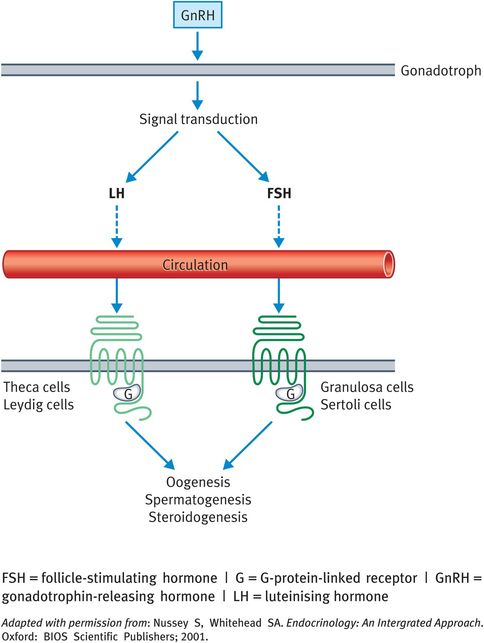
The gonadotrophins, such as FSH, LH and hCG, are glycoproteins (molecular weights approximately 30 000 Dalton) made up of a gonadotrophin-specific alpha subunit and a beta subunit that confers biological specificity to each hormone. LH and FSH are secreted from gonadotrophs in the anterior pituitary gland. These cells make up 10–15% of the anterior pituitary cells (Figure 16.3). Their receptors are typical G-protein-linked proteins. Receptor activation by gonadotrophin binding stimulates adenylate cyclase, resulting in a rise in cyclic adenosine monophosphate (cAMP) levels. FSH and LH are important regulators of steroidogenesis in the gonads. In the testis, LH acts on the interstitial or Leydig cells, whereas FSH acts on the Sertoli cells. In the ovary, each hormone acts on more than one cell type. There are numerous isoforms of circulating LH and FSH and biological potency depends on the degree and sites of glycosylation and the electrical charge of the molecule. Thus, different assays of LH and FSH concentrations may not always give a true index of biological activity.
Actions and regulation of gonadotrophins
Posterior Pituitary Gland
AVP and OXY are small peptides with strong structural homologies. They consist of nine amino acids arranged in a ring structure with a short ‘tail’. AVP is mainly synthesised in the supraoptic nuclei and to a lesser extent the paraventricular nuclei, whereas the reverse is true for OXY. The hormones synthesised in the large (or magnocellular) neurosecretory cells of these nuclei are transferred to the posterior pituitary gland, whereas those synthesised in the smaller (or parvocellular) neurons control the hormone secretions of the anterior pituitary gland.
Like all other peptide and protein hormones, AVP and OXY are synthesised as large prohormones. Once packaged into secretory granules, the AVP and OXY prohormones pass by axonal flow to the nerve terminals (Herring bodies) in the neurohypophysis. During this passage, the prohormones are cleaved into the biologically active AVP or OXY molecules and a larger polypeptide fragment. Neurophysin II is the product cleaved from the vasopressin prohormone, neurophysin I is cleaved from the OXY prohormone. Both neurophysins are co-secreted with the active hormones upon electrical activation of the neurosecretory cells.
Arginine vasopressin
AVP derives its name from the first action ascribed to it nearly 100 years ago: an increase in systemic blood pressure (it is a pressor agent). The actions of AVP on water balance in the kidney (where it is effective in very low concentrations) gave rise to an alternative name, antidiuretic hormone, and the two names tend to be used interchangeably. AVP has three known receptors and acts in concert with aldosterone and atrial natriuretic peptide to control blood volume and pressure. However, this chapter will consider exclusively the actions of AVP.
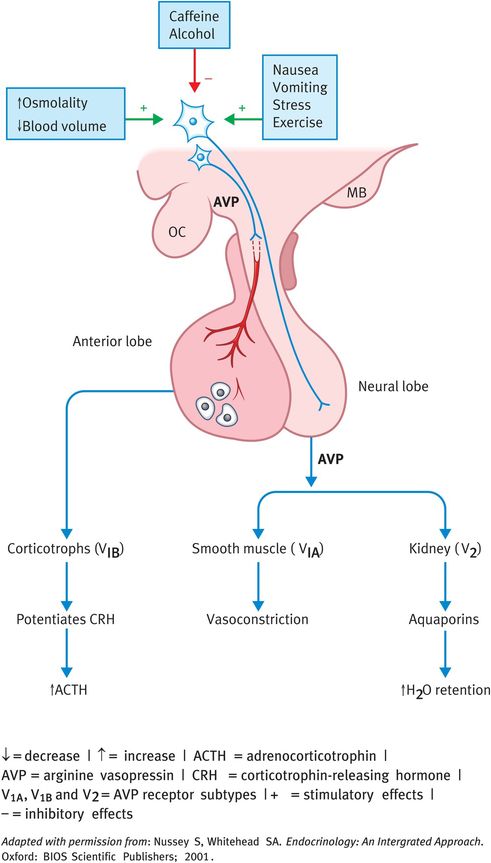
In the kidney, AVP acts on the G-protein-linked V2 receptors on the capillary (basal) side of the distal convoluted and collecting ducts and stimulates the synthesis of cAMP (Figure 16.5) cAMP activates a kinase on the luminal (apical) side of the ducts, which initiates a series of events culminating in the insertion of water channels, known as aquaporins, into the luminal membrane. Water passes into the collecting-duct cells and, by osmosis, across the basal membrane into the interstitial fluid and hence back into the circulation. There is also an osmotic gradient (set up by the counter-current activity of the loop of Henle) from the cortex to the medulla of the kidney. Thus, as the collecting ducts pass through the cortex to the medulla, increasing amounts of solute-free water (also termed free water) can be reabsorbed by the osmotic gradient. This is governed by the aquaporins and hence AVP.
Increased osmolality of the extracellular fluids stimulates AVP release (Figure 16.5). Changes in osmolality are detected by osmoreceptors. Osmotically sensitive cells were first described in the hypothalamus, but it is now known that there are additional osmoreceptors in the circumventricular organs and in systemic viscera. These are not only important in regulating AVP secretion but also in stimulating thirst. As a result, AVP induces an increase in water retention and a reduction in serum osmolality.
Another potent stimulus to AVP secretion is a reduction in effective blood volume; in fact, AVP secretion is stimulated by a volume reduction of just 5–10%. This is controlled by so-called stretch receptors that detect changes in blood volume or pressure. These include the baroreceptors and other receptors in the cardiovascular system. Thus, for example after a haemorrhage, AVP secretion will be stimulated and water will be retained to increase blood volume. The reverse occurs in response to an increase in blood volume or pressure.
Other stimulants of AVP secretion include emotional stress, pain and a variety of drugs. Nausea and vomiting are also extremely potent stimulators of AVP release. The effects of these stimuli may be important in situations such as the postoperative state, affecting water balance. By contrast, alcohol is a potent inhibitor of AVP release (as little as 30–90 ml of whiskey is sufficient) resulting in inappropriate dehydration and some of the symptoms associated with a hangover. The preventative measure is to drink a restorative volume of water after excess ethanol consumption.
Apart from its primary role in water conservation, AVP is also important in maintaining blood pressure after a haemorrhage as a result of its vasopressive effects on arteriolar smooth muscle. Acting on smooth muscle cells, it induces vasoconstriction via calcium and phospholipase-C-generated second messengers. The final established role of vasopressin is the potentiation of CRH action on pituitary corticotrophs.
Oxytocin
OXY is neuropeptide and exerts a wide spectrum of central and peripheral effects as neurohormone, neurotransmitter or neuromodulator. OXY is centrally produced in magnocellular neurons in hypothalamus. OXY is also produced in peripheral tissues; for example, uterus, placenta, amnion, corpus luteum, testis and heart. OXY receptors have also been identified in other tissues, including the kidney, heart, thymus, pancreas and adipocytes. The major action of OXY is in lactation, when, through a neuroendocrine reflex, it initiates the let-down of milk by inducing contractions of the myoepithelial cells surrounding the alveoli of the mammary gland. In animals, there is evidence that OXY has a major role in parturition, but in humans there is much less evidence to support this role. OXY does, however, have a contributing role in that it induces powerful contractions of the uterine muscle. Thus, synthetic OXY is widely used therapeutically in obstetrics, not only to induce labour but also to reduce postpartum bleeding.
OXY can have stimulatory effects at the AVP V2 receptor in the kidney. There is no naturally occurring disease associated with excess OXY secretion but in obstetric situations, when given in high doses as an intravenous infusion 5% dextrose, it can cause water retention and iatrogenic hyponatraemia.
Hypothalamic and pituitary control of puberty
Puberty is defined as the stage of physical maturation in which an individual becomes physiologically capable of sexual reproduction. It is associated with marked endocrine changes, including an increase in growth rate, skeletal changes (such as increase in hip width in girls), increase in fat and muscle and profound psychological changes.
Physiology of Puberty
The onset of puberty is characterised by an increase in the secretions of LH and FSH that is driven by GnRH release from the neurons that are diffusely situated in the arcuate nucleus and other nuclei of the hypothalamus. Before the onset of puberty, both LH and FSH are secreted in very small amounts (with low concentrations in peripheral blood) and there is no apparent stimulation of the gonads. However, as puberty approaches, the amplitudes of LH and FSH pulsatile secretions (and hence the mean secretion rates) increase and the nocturnal rise in LH secretion is amplified. It is notable that this nocturnal rhythm is specific to puberty as it disappears in adulthood. The gonad itself does not seem necessary for the changes to occur and thus it must be concluded that the brain is in some way programmed to produce more GnRH and hence LH and FSH.
The increasing circulating concentrations of LH and FSH stimulate pubertal development of the gonads and thus an increase in the output of sex steroids. These, together with adrenal androgens, induce the physical changes that occur at this time (Figures 16.4 and 16.5).
Actions and regulation of arginine vasopressin
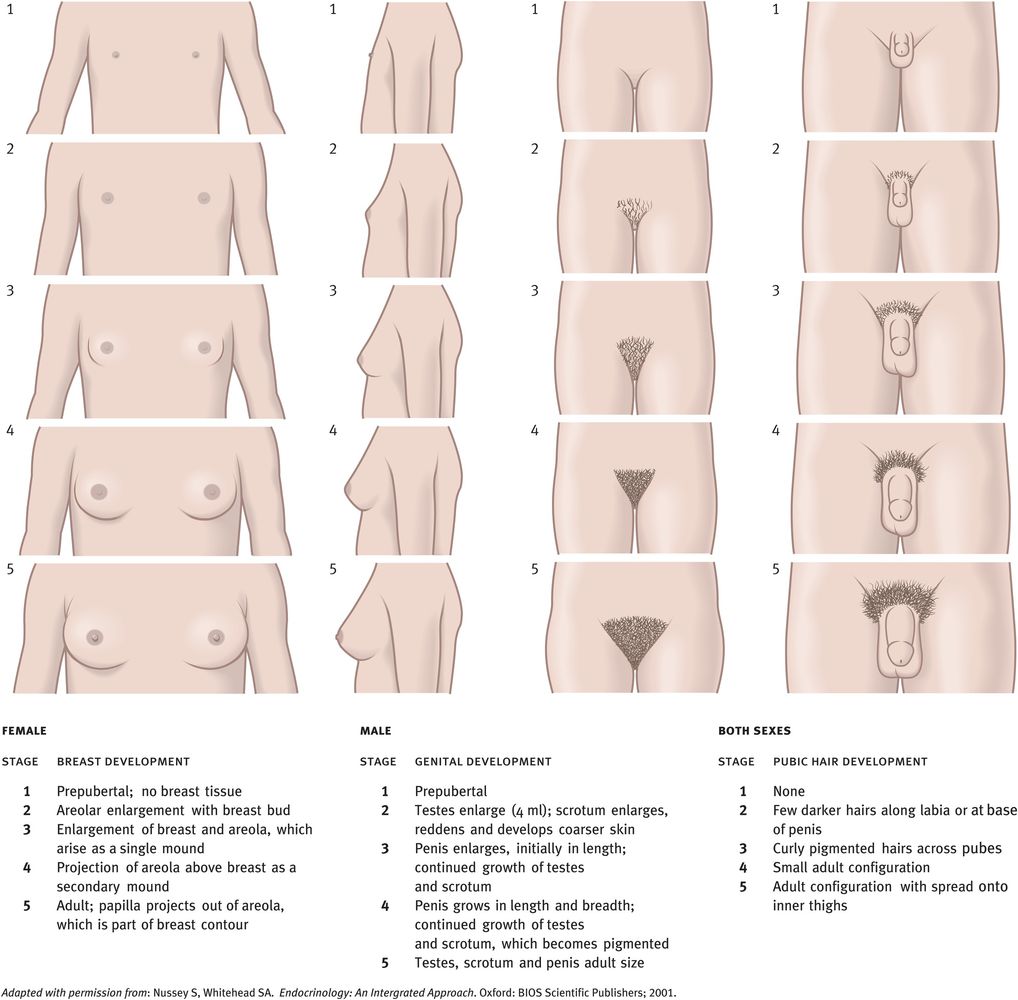
In boys, the earliest sign of increasing LH and FSH secretions is an increase in testicular size (a volume increase of more than 4 ml or a length increase of more than 2.5 cm). In girls, thelarche (breast development) is a sensitive indicator of LH- and FSH-stimulated ovarian steroid secretions. Before the menarche, however, estradiol secretion fluctuates widely, probably reflecting waves of follicular development in which the follicles fail to reach the ovulatory stage. Estrogen stimulates growth of the uterus but menarche does not occur until the estrogens have stimulated sufficient uterine growth such that their withdrawal causes the first menstruation. The onset of menstruation is such a milestone in the development of girls that primary amenorrhoea can be relied upon to indicate underlying pathology. The growth of axillary and pubic hair in both sexes is a consequence of increased concentrations of not only of gonadal steroids (gonadarche) but also of adrenal steroids (adrenarche).
Precocious Puberty
The changes during puberty normally follow a reliable pattern that is sometimes called consonance. If these changes happen early (before the age of 8 years in girls and before the age of 9 years in boys) puberty is considered to be precocious. However, recent studies indicate that signs of early puberty (breasts and pubic hair) are often present in girls (particularly black girls) aged 6–8 years. Central precocious puberty is gonadotropin-dependent, is the early maturation of the entire hypothalamo–pituitary–gonadal (HPG) axis with the full spectrum of physical and hormonal changes of puberty and is idiopathic in 90% of girls. It can be associated with central nervous system abnormalities such as tumours, brain injury or congenital brain anomalies. Peripheral precocious puberty (pseudopuberty) is much less common and refers to conditions in which increased production of sex steroids is gonadotropin-independent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree