Chapter 86 Hypoglycemia
Glucose has a central role in fuel economy and is a source of energy storage in the form of glycogen, fat, and protein (Chapter 81). Glucose, an immediate source of energy, provides 38 mol of adenosine triphosphate (ATP) per mol of glucose oxidized. It is essential for cerebral energy metabolism because it is usually the preferred substrate and its utilization accounts for nearly all the oxygen consumption in the brain. Cerebral glucose uptake occurs through a glucose transporter molecule or molecules that are not regulated by insulin. Cerebral transport of glucose is a carrier-mediated, facilitated diffusion process that is dependent on blood glucose concentration. Deficiency of brain glucose transporters can result in seizures because of low cerebral and cerebrospinal fluid (CSF) glucose concentrations (hypoglycorrhachia) despite normal blood glucose levels. To maintain the blood glucose concentration and prevent it from falling precipitously to levels that impair brain function, an elaborate regulatory system has evolved.
Definition
In neonates, there is not always an obvious correlation between blood glucose concentration and the classic clinical manifestations of hypoglycemia. The absence of symptoms does not indicate that glucose concentration is normal and has not fallen to less than some optimal level for maintaining brain metabolism. There is evidence that hypoxemia and ischemia may potentiate the role of hypoglycemia in causing permanent brain damage. Consequently, the lower limit of accepted normality of the blood glucose level in newborn infants with associated illness that already impairs cerebral metabolism has not been determined (Chapter 101). Out of concern for possible neurologic, intellectual, or psychologic sequelae in later life, most authorities recommend that any value of blood glucose <50 mg/dL in neonates be viewed with suspicion and vigorously treated. This is particularly applicable after the initial 2-3 hr of life, when glucose normally has reached its nadir; subsequently, blood glucose levels begin to rise and achieve values of 50 mg/dL or higher after 12-24 hr. In older infants and children, a whole blood glucose concentration of <50 mg/dL (10-15% higher for serum or plasma) represents hypoglycemia.
Substrate, Enzyme, and Hormonal Integration of Glucose Homeostasis
In the Newborn (Chapter 101)
In the early postnatal period, responses of the endocrine pancreas favor glucagon secretion so that blood glucose concentration can be maintained. These adaptive changes in hormone secretion are paralleled by similarly striking adaptive changes in hormone receptors. Key enzymes involved in glucose production also change dramatically in the perinatal period. Thus, there is a rapid fall in glycogen synthase activity and a sharp rise in phosphorylase after delivery. Similarly, the amount of rate-limiting enzyme for gluconeogenesis, phosphoenolpyruvate carboxykinase, rises dramatically after birth, activated in part by the surge in glucagon and the fall in insulin. This framework can explain several causes of neonatal hypoglycemia based on inappropriate changes in hormone secretion and unavailability of adequate reserves of substrates in the form of hepatic glycogen, muscle as a source of amino acids for gluconeogenesis, and lipid stores for the release of fatty acids. In addition, appropriate activities of key enzymes governing glucose homeostasis are required (see Fig. 81-1).
In Older Infants and Children
Hypoglycemia in older infants and children is analogous to that of adults, in whom glucose homeostasis is maintained by glycogenolysis in the immediate postfeeding period and by gluconeogenesis several hours after meals. The liver of a 10 kg child contains 20-25 g of glycogen, which is sufficient to meet normal glucose requirements of 4-6 mg/kg/min for only 6-12 hr. Beyond this period, hepatic gluconeogenesis must be activated. Both glycogenolysis and gluconeogenesis depend on the metabolic pathway summarized in Figure 81-1. Defects in glycogenolysis or gluconeogenesis may not be manifested in infants until the frequent feeding at 3-4 hr intervals ceases and infants sleep through the night, a situation usually present by 3-6 mo of age. The source of gluconeogenic precursors is derived primarily from muscle protein. The muscle bulk of infants and small children is substantially smaller relative to body mass than that of adults, whereas glucose requirements/unit of body mass are greater in children, so the ability to compensate for glucose deprivation by gluconeogenesis is more limited in infants and young children, as is the ability to withstand fasting for prolonged periods. The ability of muscle to generate alanine, the principal gluconeogenic amino acid, may also be limited. Thus, in normal young children, the blood glucose level falls after 24 hr of fasting, insulin concentrations fall appropriately to levels of <5-10 µU/mL, lipolysis and ketogenesis are activated, and ketones may appear in the urine.
The switch from glycogen synthesis during and immediately after meals to glycogen breakdown and later gluconeogenesis is governed by hormones, of which insulin is of central importance. Plasma insulin concentrations increase to peak levels of 50-100 µU/mL after meals, which serve to lower the blood glucose concentration through the activation of glycogen synthesis, enhancement of peripheral glucose uptake, and inhibition of glucose production. In addition, lipogenesis is stimulated, whereas lipolysis and ketogenesis are curtailed. During fasting, plasma insulin concentrations fall to ≤5-10 µU/mL, and together with other hormonal changes, this fall results in activation of gluconeogenic pathways (see Fig. 81-1). Fasting glucose concentrations are maintained through the activation of glycogenolysis and gluconeogenesis, inhibition of glycogen synthesis, and activation of lipolysis and ketogenesis. It should be emphasized that a plasma insulin concentration of >5 µU/mL, in association with a blood glucose concentration of ≤40 mg/dL (2.2 mM), is abnormal, indicating a hyperinsulinemic state and failure of the mechanisms that normally result in suppression of insulin secretion during fasting or hypoglycemia.
Clinical Manifestations (Chapter 101)
Clinical features generally fall into 2 categories. The 1st includes symptoms associated with the activation of the autonomic nervous system and epinephrine release, usually seen with a rapid decline in blood glucose concentration (Table 86-1). The 2nd category includes symptoms due to decreased cerebral glucose utilization, usually associated with a slow decline in blood glucose level or prolonged hypoglycemia (see Table 86-1). Although these classic symptoms occur in older children, the symptoms of hypoglycemia in infants may be subtler and include cyanosis, apnea, hypothermia, hypotonia, poor feeding, lethargy, and seizures. Some of these symptoms may be so mild that they are missed. Occasionally, hypoglycemia may be asymptomatic in the immediate newborn period. Newborns with hyperinsulinemia are often large for gestational age; older infants with hyperinsulinemia may eat excessively because of chronic hypoglycemia and become obese. In childhood, hypoglycemia may present as behavior problems, inattention, ravenous appetite, or seizures. It may be misdiagnosed as epilepsy, inebriation, personality disorders, hysteria, and retardation. A blood glucose determination should always be performed in sick neonates, who should be vigorously treated if concentrations are <50 mg/dL. At any age level, hypoglycemia should be considered a cause of an initial episode of convulsions or a sudden deterioration in psychobehavioral functioning.
Table 86-1 MANIFESTATIONS OF HYPOGLYCEMIA IN CHILDHOOD
FEATURES ASSOCIATED WITH ACTIVATION OF AUTONOMIC NERVOUS SYSTEM AND EPINEPHRINE RELEASE*
FEATURES ASSOCIATED WITH CEREBRAL GLUCOPENIA
* Some of these features will be attenuated if the patient is receiving β-adrenergic blocking agents.
Many neonates have asymptomatic (chemical) hypoglycemia. The incidence of symptomatic hypoglycemia is highest in small for gestational age infants (Fig. 86-1). The exact incidence of symptomatic hypoglycemia has been difficult to establish because many of the symptoms in neonates occur together with other conditions such as infections, especially sepsis and meningitis; central nervous system anomalies, hemorrhage, or edema; hypocalcemia and hypomagnesemia; asphyxia; drug withdrawal; apnea of prematurity; congenital heart disease; or polycythemia.
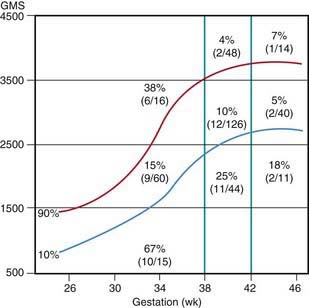
Figure 86-1 Incidence of hypoglycemia by birthweight, gestational age, and intrauterine growth.
(From Lubchenco LO, Bard H: Incidence of hypoglycemia in newborn infants classified by birthweight and gestational age, Pediatrics 47:831–838, 1971.)
Classification of Hypoglycemia in Infants and Children
Classification is based on knowledge of the control of glucose homeostasis in infants and children (Table 86-2).
Table 86-2 CLASSIFICATION OF HYPOGLYCEMIA IN INFANTS AND CHILDREN
NEONATAL TRANSIENT HYPOGLYCEMIA
Associated with Inadequate Substrate or Immature Enzyme Function in Otherwise Normal Neonates
Transient Neonatal Hyperinsulinism Also Present in:
NEONATAL, INFANTILE, OR CHILDHOOD PERSISTENT HYPOGLYCEMIAS
Hormonal Disorders
Counter-Regulatory Hormone Deficiency
Glycogenolysis and Gluconeogenesis Disorders
Lipolysis Disorders
Fatty Acid Oxidation Disorders
OTHER ETIOLOGIES
Substrate-Limited
Liver Disease
Amino Acid and Organic Acid Disorders
Systemic Disorders
GSD, glycogen storage disease; HI, hyperinsulinemia; KATP, regulated potassium channel.
Neonatal, Transient, Small for Gestational Age, and Premature Infants (Chapter 101)
The estimated incidence of symptomatic hypoglycemia in newborns is 1-3/1,000 live births. This incidence is increased severalfold in certain high-risk neonatal groups (see Table 86-2 and Fig. 86-1). The premature and small for gestational age (SGA) infants are vulnerable to the development of hypoglycemia. The factors responsible for the high frequency of hypoglycemia in this group, as well as in other groups outlined in Table 86-2, are related to the inadequate stores of liver glycogen, muscle protein, and body fat needed to sustain the substrates required to meet energy needs. These infants are small by virtue of prematurity or impaired placental transfer of nutrients. Their enzyme systems for gluconeogenesis may not be fully developed. Transient hyperinsulinism responsive to diazoxide has also been reported as contributing to hypoglycemia in asphyxiated, SGA, and premature newborn infants. This form of hyperinsulinism associated with perinatal asphyxia, intrauterine growth restriction (IUGR), maternal toxemia and other perinatal stressors, is probably the most common cause of hyperinsulinemic hypoglycemia in neonates and may be quite severe. In most cases, the condition resolves quickly, but it may persist to 7 mo of life or more. A genetic cause of this form of dysregulated insulin secretion has not been established.
Infants Born to Diabetic Mothers (Chapter 101)
Mothers whose diabetes has been well controlled during pregnancy, labor, and delivery generally have infants near normal size who are less likely to develop neonatal hypoglycemia and other complications formerly considered typical of such infants (Chapter 101). In supplying exogenous glucose to these hypoglycemic infants, it is important to avoid hyperglycemia that evokes a prompt exuberant insulin release, which may result in rebound hypoglycemia. When needed, glucose should be provided at continuous infusion rates of 4-8 mg/kg/min, but the appropriate dose for each patient should be individually adjusted. During labor and delivery, maternal hyperglycemia should be avoided because it results in fetal hyperglycemia, which predisposes to hypoglycemia when the glucose supply is interrupted at birth. Hypoglycemia persisting or occurring after 1 wk of life requires an evaluation for the causes listed in Table 86-2.



