Hyperandrogenism is the excess production and/or action of the male hormones testosterone and dihydrotestosterone, and their precursors—androstenedione and dehydroepiandrosterone. Hyperandrogenism affects approximately 5% of reproductive age women. The most common causes of hyperandrogenism include polycystic ovary syndrome (PCOS), idiopathic hirsutism, nonclassical adrenal hyperplasia (NCAH) due to a 21-hydroxylase deficiency and ovarian or adrenal androgen-secreting tumors. For women affected by hyperandrogenism, their chief complaints may include oligomenorrhea or amenorrhea, hirsutism, anovulatory infertility, and obesity. Hyperandrogenic women are at increased risk for type 2 diabetes, gestational diabetes, and the metabolic syndrome, including dyslipidemia and hypertension. Hyperandrogenism often begins insidiously early in puberty, and slowly and stealthily becomes progressively more severe until the full phenotype, including hirsutism and oligomenorrhea, emerges firmly established in late adolescence.
1 Hyperandrogenism is associated with both reproductive and metabolic abnormalities, including increased pituitary secretion of luteinizing hormone (LH) and insulin resistance in muscle and adipose tissue. Fortunately, most cases of hyperandrogenism are successfully treated either by using estrogen-progestin contraceptives to suppress the excess LH secretion and ovarian androgen overproduction or by using an insulin sensitizer to reduce the hyperinsulinemia that contributes to ovarian androgen overproduction.
ENDOCRINE BIOLOGY OF HYPERANDROGENISM
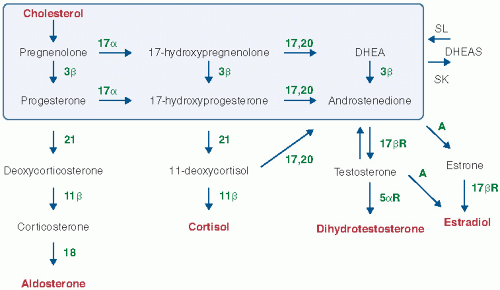
Androgens are those steroids that stimulate growth of the male secondary sex glands, such as the prostate. Androgens bind to the intracellular androgen receptor and alter the transcription of specific genes involved in the development of the male secondary sex phenotype. Testosterone (T) and dihydrotestosterone (DHT) are the biologically active androgens. Androstenedione (A) and dehydroepiandrosterone sulfate (DHEA-S) are important androgen precursors but have little inherent androgen activity. Hyperandrogenism is the state of increased androgen production and action. In most cases of hyperandrogenism, the ovary, the adrenal, and the pilosebaceous unit contribute to the androgen overproduction and action.
Two-Gonadotropin, Two-Cell Theory: Dual Defects Causing Hyperandrogenism
The ovarian follicle contains two main cell types: theca cells line the outer surface of the follicle and granulosa cells are within the inner core of the follicle. Theca cells express LH receptors and convert pregnenolone to androstenedione (
Fig. 3.1). Granulosa cells from small follicles express follicle-stimulating hormone (FSH) receptors and convert androstenedione to estradiol, the main biologically active estrogen. In many cases of hyperandrogenism, there is an imbalance in the biological effects of LH and FSH, with too much LH effect and too little FSH effect. This results in excess secretion of androgen and insufficient conversion of estrogen to androgen, resulting in an androgen-dominant ovarian environment. When the gonadotropins LH and FSH are out of balance, the responding theca and granulosa cells are not collaboratively functioning and anovulation is the result.
All estrogen is derived from the androgen precursor, androstenedione. In the ovarian follicle from normally cycling women, there is a proper balance of LH and FSH activity resulting in the conversion of most androstenedione to estradiol and creating a follicular milieu that is dominated by estrogen and is healthy. In most cases of hyperandrogenism, the excess LH activity and the insufficient FSH results in excess accumulation of androstenedione and testosterone and insufficient production of estradiol, resulting in a follicular milieu that is dominated by androgen and is unhealthy. In these unhealthy follicles, the granulosa cells cannot grow to help the follicle to develop into a large preovulatory follicle, 18 to 25 mm in diameter. The growth of the follicle is stunted and remains in the size range of 2 to 9 mm in diameter. Little estrogen is secreted from these follicles resulting in no preovulatory surge of estradiol, which is the key factor that triggers the LH surge and ovulation. A hyperandrogenic ovarian environment is associated with disordered follicle growth, oligo- or anovulation, oligomenorrhea or amenorrhea, and increased circulating androgens.
2
Adrenal Cortisol and Androgen Secretion: A Delicate Balance
The main role of the adrenal gland is to secrete cortisol and aldosterone. However, given the organization of cortisol synthesis (
Fig. 3.1), androgens are always produced as a “spill over” product from the cortisol pathway. The normal adrenal gland is delicately balanced to produce adequate amounts of cortisol without secreting excessive androgens. Unfortunately, minor reductions in any of the enzymes in the cortisol pathway can lead to excessive production of androgen. For example, in women with NCAH due to 21-hydroxylase activity, a decreased efficiency of the enzyme 21-hydroxylase due to a genetic defect causes a build up of a cortisol precursor, 17-hydroxyprogesterone (
Fig. 3.1). Instead of 17-hydroxyprogesterone efficiently proceeding to cortisol, it is converted to androstenedione, which is then converted to testosterone and dihydrotestosterone. The partial block to cortisol synthesis results in an imbalance of the cortisol to androgen production ratio in the adrenal. The 21-hydroxylase defect is the cause of hyperandrogenism in about 5% of women with hyperandrogenism.
If adrenocorticotropic hormone (ACTH) stimulation of the adrenal is excessive, this causes an increase in both cortisol and androgen production, but the ratio of the two remains relatively normal. In Cushing disease, excessive ACTH secretion causes both excessive production of cortisol and androstenedione. Cushing disease is a rare cause of hyperandrogenism.
Dihydrotestosterone Is the Dominant Androgen in Target Tissues
In the hair follicle and other peripheral organs, androstenedione and testosterone are converted to dihydrotestosterone (DHT) by the enzyme 5α-reductase. DHT binds to the androgen receptor with an affinity 10-fold higher than that of testosterone. The importance of DHT as the dominant androgen within cells is highlighted in individuals with inherited deficiencies of 5α-reductase. Males lacking this enzyme are phenotypically female at birth because they are unable to convert testosterone to DHT and are thus unable to activate a program of male differentiation during development. All hyperandrogenic women have excessive conversion of testosterone to DHT in peripheral tissues. This occurs because elevated DHT concentration in peripheral tissues, such as hair follicles, stimulates the synthesis of more 5α-reductase resulting in more rapid and efficient conversion of testosterone to DHT, a positive feedback loop. DHT is an intracellular steroid, and very little reenters the circulation, so it is not a useful target for blood measurement.
Sex Hormone Binding Globulin: The Testosterone Trap
In the circulation, testosterone is tightly bound to sex hormone binding globulin (SHBG), weakly bound to albumin, and a small percentage is unbound (free). Testosterone bound to SHBG is unavailable to enter peripheral cells and is functionally trapped in a nonactive form. SHBG is a testosterone trap. Only the unbound and albumin-bound forms of testosterone can enter cells and be converted to the potent androgen DHT, thereby stimulating androgen effects. In normally cycling, wellestrogenized women, SHBG concentrations are remarkably high, trapping most of the circulating testosterone. In hyperandrogenic women, SHBG levels are remarkably low, allowing the testosterone to freely enter cells and be converted to DHT. Obesity and insulin resistance independently contribute to a decrease in SHBG concentration. Measurement of SHBG is a reasonable proxy for identifying hyperandrogenic women and can be used in conjunction with total testosterone to calculate a “free androgen index.”
Obesity Is Associated With Increased Circulating Androgens and Decreased Sex Hormone Binding Globulin
Obesity and insulin resistance cause a dual defect in androgen biology, increasing testosterone production and reducing SHBG concentrations resulting in an additive increase in free testosterone, which is then available to be converted to DHT in peripheral tissues.
3 Obesity is associated with insulin resistance in muscle, liver, and adipose tissues, resulting in a compensatory hyperinsulinemia. Insulin stimulates ovarian production of androgens and reduces SHBG synthesis by the liver.
Cigarette Smoking Is Associated With Increased Circulating Androgens
Constituents of cigarette smoke—nicotine and anabasine— and their metabolites, including cotinine, disrupt the delicate balance of adrenal cortisol and androgen production by increasing ACTH production and by inhibiting the 21-hydroxylase enzyme, resulting in more androgen production for every molecule of cortisol produced. Women of all ages are susceptible to this effect. Women who smoke have approximately 15% greater androgen levels than women who do not smoke.
4
Suppressing Luteinizing Hormone Secretion Decreases Circulating Androgens
As noted earlier, the dual defect in gonadotropins— excess LH and insufficient FSH—results in an imbalance in the ovarian follicle causing excess androstenedione production and insufficient estrogen production resulting in a hyperandrogenic state. Any treatment that lowers LH production will result in a decrease in ovarian androgen production. In parallel, any treatment that increases FSH production or action can overcome the relative LH excess and convert the excess androgen to estrogen, often restoring a normal estrogen environment to the follicle, allowing it to grow and ovulate.
HYPERANDROGENISM: REPRODUCTIVE AND METABOLIC ABNORMALITIES
Most hyperandrogenic women have both reproductive and metabolic abnormalities. The reproductive abnormalities include the following: (a) Excess LH and insufficient FSH secretion, caused in part by an excess secretion of hypothalamic gonadotropin-releasing hormone (GnRH). (b) Excess ovarian production of androgen and insufficient ovarian production of estradiol. (c) Stunted growth of ovarian follicles resulting in follicles in the range of 2 to 9 mm in diameter. (d) In the absence of a large, 18- to 25-mm healthy preovulatory follicle, the estradiol surge and the LH surge cannot occur resulting in oligo- or anovulation. (e) The excess ovarian androgen secretion results in decreased SHBG, increased free testosterone, increased testosterone entry into peripheral cells, and excessive conversion of testosterone to DHT, the most potent intracellular androgen. This causes hirsutism and in some women other signs of androgen excess, such as alopecia. (f) The oligo- and anovulation of hyperandrogenism can cause anovulatory infertility.
The metabolic abnormalities of hyperandrogenic women include the following: (a) Insulin resistance in both lean and obese hyperandrogenic women in muscle, liver, and adipose tissue. Insulin resistance is typically more severe in obese hyperandrogenic women. (b) An increased prevalence of impaired glucose tolerance, impaired fasting glucose, and diabetes. (c) An increased prevalence of gestational diabetes if pregnancy occurs. (d) An increased prevalence of the metabolic syndrome, especially in obese hyperandrogenic women. The phenotype of the metabolic syndrome includes decreased levels of high-density lipoprotein cholesterol (HDL-C), increased fasting triglycerides, increased fasting glucose (greater than 100 mg/dL), increased waist circumference and hypertension. (e) Evidence for endothelial inflammation and accelerated atherosclerosis.
The pathophysiology of insulin resistance associated with hyperandrogenism is not well characterized. Disorders of oxidative phosphorylation, insulin receptor defects, and intracellular postreceptor defects in insulin signaling may all contribute. In hyperandrogenic women, the ovarian theca appears to be stimulated by insulin to produce androstenedione and testosterone. Consequently, hyperinsulinemia caused by insulin resistance conspires with elevated LH levels to cause excessive ovarian androgen secretion.
HYPERANDROGENISM: DIFFERENTIAL DIAGNOSIS
The main causes of hyperandrogenism are (a) the PCOS, (b) idiopathic hirsutism, (c) NCAH due to 21-hydroxylase deficiency, (d) adrenal and ovarian androgen-secreting tumors, and (e) other endocrine causes of hyperandrogenism including Cushing disease or acromegaly. In large case series, the relative frequency of these four causes of hyperandrogenism are 80% PCOS, 15% idiopathic hirsutism, 4% NCAH and less than 1% adrenal and ovarian tumors, and less than 1% Cushing disease or acromegaly.
Currently, there are three competing approaches to the diagnosis of PCOS (
Table 3.1). The author uses the National Institutes of Health (NIH) consensus criteria that require the presence of BOTH hyperandrogenism and oligo- or anovulation as manifested by oligomenorrhea or amenorrhea.
5 Imaging studies to document the polycystic ovary morphology are not required in this diagnostic system. Hyperandrogenism must be present.
The Rotterdam criteria require the presence of two of three findings: hyperandrogenism, oligo- or anovulation, or an imaging study indicating a polycystic ovary morphology.
6 To consistently use the Rotterdam criteria, all women undergoing a diagnostic evaluation for hyperandrogenism need high-resolution sonography of the ovaries. This results in an increase in resource use with little evidence for clinical benefit. Also, the Rotterdam criteria permit the diagnosis of PCOS even if the woman is not hyperandrogenic, but hyperandrogenism is the sine qua non of PCOS. The Rotterdam criteria results in a higher prevalence of PCOS than the NIH consensus criteria because of its relaxed diagnostic criteria.
The criteria of the Androgen Excess Society
requires the presence of hyperandrogenism but allows for the use of either oligo-ovulation
or a polycystic ovary morphology as evidence of ovarian dysfunction.
7Idiopathic hirsutism is diagnosed when the patient self-reports excess facial hair growth, but she has regular ovulatory menstrual cycles. It is likely that many women with idiopathic hirsutism have a subtle increase in ovarian, adrenal, and/or hair follicle androgen production. A few may have a mild form of PCOS and would meet the Rotterdam criteria. However, if a woman has regular ovulatory cycles, it is unlikely that she has a significant endocrinopathy.
NCAH due to 21-hydroxylase deficiency has a phenotype that is identical to the phenotype of PCOS: hyperandrogenism and oligo-ovulation are uniformly present. Consequently, the only method to identify NCAH due to 21-hydroxylase deficiency is to measure an early morning 17-hydroxyprogesterone level, which is always elevated in NCAH and never elevated in PCOS.
Ovarian and adrenal tumors usually present with marked elevation in circulating total testosterone (greater than 150 ng/dL and often greater than 200 ng/dL) and signs of virilization including deepening of the voice register, increased upper body muscle mass, clitoromegaly, and/or alopecia. The screening test for Cushing disease is a 24-hour urine cortisol measurement. A screening test for acromegaly is measurement of circulating insulin-like growth factor-1 (IGF-1).
DIAGNOSIS OF HYPERANDROGENISM
Self-Reported Hirsutism
Historically, hirsutism was assessed using the modified Ferriman-Gallwey scoring system that uses nine separate body sites, each scored 1 to 4 and summed to a single total number to measure the severity of hirsutism. The Endocrine Society now recommends that clinicians focus their attention on the patient’s self-report of hirsutism, rather than a quantitative assessment of hirsutism on physical examination.
Status of Menstrual Cycles
Women with PCOS typically report irregular menstrual cycles starting in adolescence. Many never have regular ovulatory cycles. Women with idiopathic hirsutism always have regular ovulatory cycles. Women with ovarian or adrenal androgen-secreting tumors are typically amenorrheic.
Physical Examination
Key points in the physical examination of hyperandrogenic women are presented in
Table 3.2.
Laboratory Issues
Key laboratory tests are outlined in
Table 3.2.
Total and Free Testosterone
Either total or free testosterone or both can be measured. Serum testosterone provides the best laboratory estimate of the severity of androgen overproduction. Total testosterone measurement is performed by all clinical laboratories and is a reasonably well-standardized test, especially in the range greater than 1.5 ng/mL. The measurement of total testosterone is usually less expensive than free testosterone measurement. Many women with PCOS have a total testosterone level in the upper end of the normal range (about 0.60 to 0.80 ng/mL). This range is not well standardized among clinical laboratories. If the total testosterone level is greater than 2 ng/mL (200 ng/dL), the patient probably has ovarian stromal hyperthecosis or an adrenal or ovarian tumor
and needs a detailed evaluation, which should include imaging studies of the ovary and adrenal glands. The free testosterone measurement is more sensitive in detecting mild androgen overproduction. The free testosterone assay is not well standardized among laboratories and is more expensive than a total testosterone assay. The author does not usually order free testosterone measurements.
17-Hydroxyprogesterone
Approximately 4% of women who present with hyperandrogenism and oligo-ovulation or anovulation have NCAH resulting from a 21-hydroxylase deficiency. The prevalence of this genetic disorder varies markedly among different ethnic groups, from below 1% in Hispanic populations to as high as 8% in Ashkenazi Jewish populations and even higher in Eskimo populations. The decision to screen for the disorder depends on the cost-benefit assessment of detection and the baseline prevalence of the disorder in the patient’s ethnic group. If the 17-hydroxyprogesterone level at 8 AM is greater than 2 ng/mL, the patient probably has NCAH resulting from a 21-hydroxylase deficiency. This diagnosis can be confirmed by a 60-minute ACTH stimulation test. The test uses a form of synthetic ACTH (cosyntropin) that contains the first 24 of the 39 amino acids of natural ACTH; 0.25 mg is given intravenously or intramuscularly, and the 17-hydroxyprogesterone level is measured 60 minutes later. A post-ACTH 17-hydroxyprogesterone level greater than 10 ng/mL confirms the diagnosis of NCAH resulting from a 21-hydroxylase deficiency.
Dehydroepiandrosterone Sulfate
DHEA-S, an androgen prohormone that can be converted to testosterone in the periphery, is secreted almost exclusively by the adrenal glands. The normal DHEA-S level in premenopausal women is 0.12 to 5.35 mcg/dL. A DHEA-S level above 10.70 mcg/dL—that is, more than twice the upper limit of normal—should raise concern for a possible adrenal tumor.
Prolactin, Thyroid-Stimulating Hormone, and Follicle-Stimulating Hormone
If the patient has amenorrhea, the laboratory workup should include an assessment of serum prolactin level to rule out a prolactin-secreting pituitary tumor. In an amenorrheic woman, most clinicians also routinely measure serum FSH and thyroid-stimulating hormone (TSH) levels.
Luteinizing Hormone and Follicle-Stimulating Hormone
The measurement of serum LH presents a special problem in the laboratory evaluation of PCOS. In the research setting—using multiple serum LH measurements (every 10 minutes for at least 8 hours) and a precise and reliable LH assay—elevated LH levels can be documented in more than 95% of women with PCOS. However, because LH secretion is pulsatile and the standard commercial assays are not as precise as research assays, measurement of LH in clinical practice is of little use. An elevated LH level is reasonably specific for PCOS, provided the sample was not taken during a preovulatory LH surge. A normal LH value does not necessarily exclude PCOS, however, because the test sample may have been drawn when the patient was at the nadir of an LH pulse. Another important point is that as body mass index (BMI) increases, the normal range for LH decreases.
8 Nomograms that control serum LH for BMI are not widely available. Many women with PCOS have an LH:FSH ratio greater than 2. The ratio of LH:FSH may be a better test for PCOS than a single LH measurement. The author does not usually order serum LH or FSH in evaluating women for hyperandrogenism.
Serum Anti-Müllerian Hormone
Anti-Müllerian hormone (AMH) concentration is elevated two-fold to three-fold in the circulation of women with PCOS compared with ovulatory women. The number of small antral follicles detected on transvaginal imaging is correlated with serum AMH concentration. Some authorities have recommended replacing the Rotterdam criteria of counting the number of small antral follicles with a serum measurement of AMH.
9