Chapter 244 Herpes Simplex Virus
Clinical Manifestations
Acute Oropharyngeal Infections
Herpes gingivostomatitis most often affects children 6 mo to 5 yr of age but is seen across the age spectrum. It is an extremely painful condition with sudden onset, pain in the mouth, drooling, refusal to eat or drink, and fever of up to 40.0-40.6°C. The gums become markedly swollen, and vesicles may develop throughout the oral cavity, including the gums, lips, tongue, palate, tonsils, pharynx, and perioral skin (Fig. 244-1). The vesicles may be more extensively distributed than typically seen with enteroviral herpangina. During the initial phase of the illness there may be tonsillar exudates suggestive of bacterial pharyngitis. The vesicles are generally present only a few days before progressing to form shallow indurated ulcers that may be covered with a yellow-gray membrane. Tender submandibular, submaxillary, and cervical lymphadenopathy is common. The breath may be foul as a result of overgrowth of anaerobic oral bacteria. Untreated, the illness resolves in 7-14 days, although the lymphadenopathy may persist for several weeks.
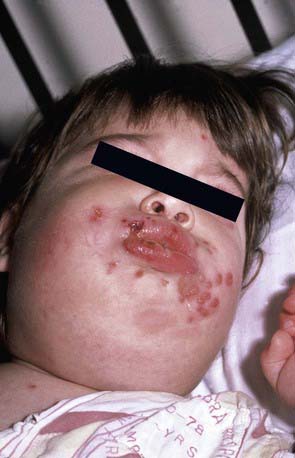
Figure 244-1 Clustered perioral vesicles and erosions in an infant with primary herpetic gingivostomatitis.
(From Schachner LA, Hansen RC, editors: Pediatric dermatology, ed 3, Philadelphia, 1988, Mosby, p 1078.)



