Hematopoiesis and Hematologic Diseases
Charles T. Quinn
George R. Buchanan
Hematologic problems are encountered daily by pediatricians caring for sick newborn infants. Alterations in the hematopoietic system most often are reactive, secondary, and even iatrogenic, but they are useful markers of underlying systemic diseases, including infection, asphyxia, and genetic and metabolic disorders. Often, these secondary hematologic problems have serious sequelae, so they must be recognized promptly and must be treated appropriately. Primary hematologic disorders, on the other hand, are rare during the newborn period. Yet, such conditions as hemophilia, immune-mediated thrombocytopenia resulting from transplacental maternal antibody, and inherited hemolytic anemia must be recognized by pediatricians so that they can initiate appropriate management, both acutely and with regard to long-term therapy and genetic counseling.
This chapter reviews the hematopoietic system in fetuses and newborn infants, focusing on common clinical problems that require differential diagnosis and management.
NORMAL DEVELOPMENTAL HEMATOPOIESIS
Hematopoiesis begins in the embryo during the third week of gestation, when blood islands in the yolk sac first produce erythrocytes and leukocytes. By the twelfth week of gestation, the liver and spleen are the predominant sites of hematopoiesis; by 30 weeks’ gestation, the bone marrow assumes its ultimate role as the major site of production of the formed elements of the blood. At the time of or shortly after birth, hematopoiesis is restricted to the bone marrow except for the pathologic states described here. At birth, large numbers of pluripotent stem cells also are present in the peripheral blood. Widespread interest has developed in collecting umbilical cord or placental blood for storage to serve as a source of donor stem cells for purposes of transplantation. Umbilical cord blood transplants, using enriched stem cells harvested from placental blood, have been used to reconstitute the bone marrow of human leukocyte antigen–matched siblings or unrelated persons suffering from bone marrow failure, genetic diseases, and leukemia. This area currently is under intense study.
Production of Red Blood Cells
The predominant cellular element in the blood is the erythrocyte, or red blood cell (RBC). The factors controlling erythropoiesis in fetuses and newborns are similar to those of older children. Pluripotent stem cells give rise to morphologically indistinct precursors committed to the erythroid lineage. Under the stimulus of erythropoietin and other humoral factors, these erythroid burst–forming units and colony-forming units give rise to identifiable erythroblasts, which proliferate, begin to synthesize hemoglobin, and mature into differentiated nucleated RBCs. In the final stages of maturation, the nucleus is extruded, and the cell enters the circulation as a young erythrocyte. The major constituent of the erythrocyte is hemoglobin, which binds oxygen in the lungs and transports it to the tissues. Hemoglobin is a tetramer consisting of two pairs of similar polypeptide chains, each attached to a heme molecule, composed of protoporphyrin and ferrous iron, the oxygen-binding site.
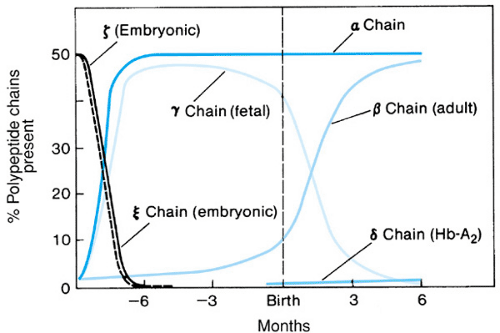
The major difference between erythropoiesis in neonates and that in older children and adults is the dynamic but poorly understood “switch” from fetal to adult hemoglobin production. The primary hemoglobin in postnatal life is hemoglobin A, consisting of two alpha chains (whose genes are encoded on chromosome 16) and two beta chains (each of which derives from a 60-kilobase-long gene complex on chromosome 11). As shown in Fig. 66.1, early in fetal life the hemoglobin tetramer contains several types of embryonic globin chains (e.g., epsilon and zeta), the synthesis of which soon declines. During the initial months of gestation, the embryonic polypeptides are replaced by gamma chains, resulting in the predominant fetal hemoglobin or hemoglobin F (alpha2- gamma2). Yet, as early as the fourteenth week of gestation, the “adult” beta globin genes are activated, beta chains are produced, and hemoglobin A (alpha2-beta2) is detectable. Alpha-chain production is sustained at a high level throughout most of fetal life. At the time of birth, gamma- and beta-chain synthesis is approximately equal, and 60% to 80% of the total hemoglobin is hemoglobin F.
Gamma globin synthesis almost ceases during the initial months of life (see Fig. 66.1). By 6 months of age, the percentage of hemoglobin F approximates that of adults (less than 2%). An increased understanding of the gamma-to-beta switch would have an impact on the treatment of a number of diseases (e.g., sickle cell anemia and beta-thalassemia) in which retention of large quantities of hemoglobin F in the erythrocyte is desirable.
Fetal hemoglobin differs from hemoglobin A in a number of ways. Hemoglobin F is resistant to both alkali and dilute acid, forming the basis for two tests for its measurement (the Kleihauer-Betke stain for fetal hemoglobin in individual cells and quantitative measurement of hemoglobin F in a hemolysate). Hemoglobin F can be also differentiated from hemoglobin A by immunologic means. It binds less avidly than does hemoglobin A to 2,3-bisphosphoglycerate, an organic phosphate in the erythrocyte important in modulating oxygen
uptake and release by hemoglobin. Therefore, the affinity of hemoglobin F for oxygen is fairly high, allowing the fetus to extract oxygen from the maternal circulation.
uptake and release by hemoglobin. Therefore, the affinity of hemoglobin F for oxygen is fairly high, allowing the fetus to extract oxygen from the maternal circulation.
The fetal RBC differs from its adult counterpart in a number of other characteristics. Fetal RBCs are larger and have higher levels of certain glycolytic enzymes, a relative deficiency of key defense mechanisms against excessive oxidation (e.g., glutathione peroxidase, catalase, methemoglobin reductase), diminished deformability, and a shorter lifespan in the circulation.
Leukocyte, Platelet, and Coagulation Protein Production
Like RBCs, granulocytes and platelets are derived from committed precursor cells in the yolk sac, liver, spleen, and then bone marrow. The developmental process and control mechanisms are similar to those noted in older children and adults. Blood coagulation factors and inhibitors, equal in importance to platelets in the fine balance of the hemostatic mechanism, generally are produced in diminished quantities during fetal life. The liver produces most blood coagulation factors and such inhibitors as antithrombin and protein C; the physiologic immaturity of the liver in the fetus and neonate results in reduced levels of most of these factors.
ANEMIA
Normal Values
Because of relative intrauterine hypoxia and the high affinity of hemoglobin F for oxygen (resulting in a shift to the left of the oxygen–hemoglobin dissociation curve), erythropoietin secretion is enhanced during fetal life. Accordingly, during the final months of gestation and at birth, values for hemoglobin and hematocrit are higher than are those for older children. Representative values are shown in Table 66.1. The mean cord blood hemoglobin concentration in term infants is 16.5 g/dL (range, 14 to 22 g/dL). Premature infants have slightly lower values. Values depend on method of delivery, site of blood sampling, postnatal age, and other factors. Capillary specimens are higher (by 1 to 2 g/dL) and generally have a wider range of hemoglobin values than that in samples obtained from the umbilical or peripheral vein. In the performance of serial hemoglobin measurements during the initial hours and days of life, such differences should be kept in mind; ideally, the same site of sampling is used consistently. Even though venous sampling is technically more difficult, it is preferred because neonatal blood is viscous (owing to the high hematocrit and reduced deformability of individual cells) and peripheral circulation in sick neonates frequently is sluggish.
The blood volume in term infants is 80 to 90 mL/kg at birth (90 to 100 mL/kg in premature infants). During the initial hours of life, a decrease in plasma volume occurs, resulting in an increase in the hemoglobin concentration as compared to cord blood values (see Table 66.1). This alteration is followed by a progressive, slow decline in the hemoglobin concentration termed the physiologic anemia of infancy. The lifespan of fetal and neonatal RBCs is approximately 80 days, as compared to 120 days in adults. Reasons for this shortened RBC survival are unclear; it is not due simply to the presence of fetal hemoglobin.
At birth, more RBCs are produced than are in older patients, as shown by the elevated reticulocyte count and the appearance of nucleated RBCs on the peripheral smear. Erythropoiesis can be accelerated further by various causes of fetal hypoxia or anemia. The usual reticulocyte count in cord blood or during the first or second day of life is 2% to 8%, and 3 to 10 nucleated RBCs per 100 leukocytes generally are present. By 3 or 4 days of life, nucleated RBCs disappear, after which their presence is always abnormal, and the reticulocyte count falls (and remains low) until 3 months of age, when recovery from physiologic anemia begins. The erythrocytes of neonates are larger than those of older children. Typically, mean cell volume is 95 to 120 fL, as compared to 70 to 85 fL in children older than several months.
TABLE 66.1. NORMAL HEMOGLOBIN VALUES IN NEWBORN INFANTS | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||
Physiologic Anemia of Infancy and Anemia of Prematurity
Shortly after birth, erythropoiesis almost ceases because of the oxygen-rich milieu and relative excess of RBCs. The progressive fall in hemoglobin values during the first several months of life in term and premature infants has been named, respectively, the physiologic anemia of infancy and the anemia of prematurity. In premature infants, the decline occurs more rapidly (with lowest values at 4 to 8 weeks, as opposed to 10 to 12 weeks in term infants), and the anemia is more severe. Factors determining the time course and severity include birth weight, perinatal complications, blood transfusion history (as premature infants who receive multiple transfusions generally exhibit a greater decline), and presence of vitamin E deficiency. Erythropoietin production during this period is relatively decreased. Nadir hemoglobin values may reach 9.5 g/dL at 3 months in term infants and 6 g/dL in 6- to 8-week-old premature infants. Recovery from physiologic anemia is heralded by a slight elevation in the reticulocyte count and a rise in hemoglobin value to levels seen throughout the remainder of infancy. To support erythropoiesis during the recovery stage of physiologic anemia, abundant iron must be available from existing stores, dietary sources, or exogenous supplements, particularly in rapidly growing premature infants, thus necessitating their higher daily iron requirement of 2 mg/kg/day, compared to 1 mg/kg/day for the term infant.
The physiologic anemia of infancy does not respond to iron or folic acid. Healthy term infants and asymptomatic, growing, premature infants require no therapy. However, apneic episodes—as well as other signs and symptoms of hypoxia, such as tachycardia, irritability, and poor feeding—have been demonstrated to be eliminated by judicious use of packed RBC transfusions and possibly by erythropoietin therapy (see later). Therefore, the anemia of prematurity may not always be physiologic or “normal.”
Differential Diagnosis of Anemia
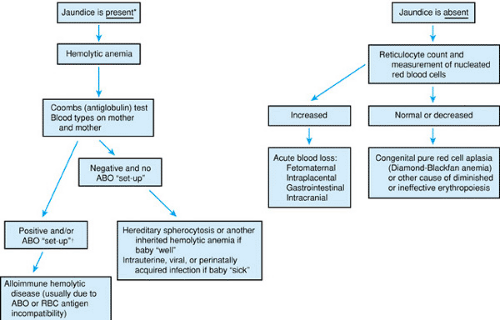
Pathophysiologic mechanisms of anemia are similar to those of older patients. Hemoglobin concentration reduction can be due to diminished production, excessive blood loss (internal or external), or hemolysis (shortened RBC lifespan). Decreased production as a primary cause of anemia is uncommon during the newborn period. It is characterized by a relatively reduced reticulocyte response and by paucity or absence of nucleated RBCs on the peripheral blood smear. Acute blood loss is common; it can be clinically obvious, but it is often occult or iatrogenic. Hemolytic anemia during the newborn period may be due either to intrinsic inherited defects in the RBC or to acquired causes. Hemolysis in newborn infants is identified easily by the marked jaundice that usually results from hepatic immaturity. Figure 66.2 depicts an algorithm of the differential diagnosis of anemia during the newborn period.
Anemia Due to Blood Loss
Anemia due to blood loss is more common during the newborn period than at any other time in childhood. Signs and symptoms relate to the duration and amount of blood lost. Acute
hemorrhage greater than 20% to 30% of the infant’s blood volume results in signs and symptoms of shock (pallor, lethargy, tachycardia, hypotension). Jaundice is absent. External blood loss occurs most commonly from the gastrointestinal tract, sometimes from an identifiable anatomic lesion (e.g., ulcer or duplication), but often without apparent cause. In instances of hematemesis or melena, whether the complication is due to swallowed maternal blood or to blood from the baby is determined by the Apt test for fetal hemoglobin (Table 66.2). Hemorrhage may occur from one twin to another or into the umbilical cord, placenta (e.g., abruption, placenta previa, or laceration during cesarean section), or maternal circulation (fetal–maternal hemorrhage). A Kleihauer-Betke or an immunohistochemical stain for fetal hemoglobin-containing RBCs in the mother may allow for an estimate of the amount of transplacental hemorrhage. This complication is not uncommon; in 1 in 100 deliveries, lost blood can be more than 20% of the baby’s blood volume, resulting in clinically significant anemia apparent at birth or manifesting later as iron deficiency. Occult blood loss of hemodynamic significance may occur also within the cranial vault of neonates because of their relatively large head size and open sutures. Bleeding in the abdominal cavity, retroperitoneal space, subcutaneous tissues (e.g., scalp), or other internal locations may result in jaundice (because of catabolism of hemoglobin from the resorbed hematomas) in addition to anemia.
hemorrhage greater than 20% to 30% of the infant’s blood volume results in signs and symptoms of shock (pallor, lethargy, tachycardia, hypotension). Jaundice is absent. External blood loss occurs most commonly from the gastrointestinal tract, sometimes from an identifiable anatomic lesion (e.g., ulcer or duplication), but often without apparent cause. In instances of hematemesis or melena, whether the complication is due to swallowed maternal blood or to blood from the baby is determined by the Apt test for fetal hemoglobin (Table 66.2). Hemorrhage may occur from one twin to another or into the umbilical cord, placenta (e.g., abruption, placenta previa, or laceration during cesarean section), or maternal circulation (fetal–maternal hemorrhage). A Kleihauer-Betke or an immunohistochemical stain for fetal hemoglobin-containing RBCs in the mother may allow for an estimate of the amount of transplacental hemorrhage. This complication is not uncommon; in 1 in 100 deliveries, lost blood can be more than 20% of the baby’s blood volume, resulting in clinically significant anemia apparent at birth or manifesting later as iron deficiency. Occult blood loss of hemodynamic significance may occur also within the cranial vault of neonates because of their relatively large head size and open sutures. Bleeding in the abdominal cavity, retroperitoneal space, subcutaneous tissues (e.g., scalp), or other internal locations may result in jaundice (because of catabolism of hemoglobin from the resorbed hematomas) in addition to anemia.
TABLE 66.2. APT TEST FOR DIFFERENTIATION OF FETAL AND ADULT (MATERNAL) HEMOGLOBIN IN STOOL OR VOMITUS | |
|---|---|
|
In sick premature infants, the most common cause of blood loss is the iatrogenic withdrawal of multiple specimens to monitor blood gases and other laboratory parameters. Such blood sampling can amount to more than 10% to 20% of the tiny infant’s blood volume during each 24-hour period and represents the most frequent indication for blood transfusions in such babies.
The treatment of anemia due to acute blood loss depends on the amount and duration of blood loss. Infants with signs of hypovolemia should receive immediate volume replacement (crystalloid, colloid, or packed RBCs). Packed RBC transfusions alone may be indicated for less severe anemia, such as that resulting from repetitive blood sampling.
Hemolytic Anemia during the Newborn Period
Destruction of RBCs intravascularly or by the mononuclear–phagocyte system (primarily spleen and liver) results in production of 32 mg of bilirubin from every 1 g of degraded hemoglobin. The physiologically immature liver of the fetus and newborn is incapable of rapidly conjugating this excess pigment. Therefore, hyperbilirubinemia, usually with clinical jaundice, accompanies all severe hemolytic states during the initial days of life. Hemolysis in the neonate, as in older patients, also is accompanied by reticulocytosis, nucleated RBCs on the peripheral blood smear, and (sometimes) characteristic RBC morphologic changes. Usually, hemolysis during the newborn period is due to RBC injury resulting from antibody binding or mechanical effects.
ABO Incompatibility
Immune-mediated hemolytic anemia results from maternally derived alloantibody directed against antigens on fetal and neonatal RBCs. In current practice, ABO incompatibility is seen most frequently. Generally, a blood group O mother produces IgG anti-A or anti-B alloantibodies that cross the placenta and bind to her infant’s type A or B erythrocytes and to other tissues that contain blood group A or B. On the other hand, in women with blood groups A or B, anti-A or anti-B alloantibodies (also called isohemagglutinins) generally are of the IgM class and do not cross the placenta. Affected babies present with jaundice during the first several days of life. Usually, anemia is absent or mild, but most patients exhibit an elevated reticulocyte count (usually 5% to 15%) and increased numbers of nucleated RBCs on the blood smear. Also characteristic on the peripheral smear are microspherocytes resulting from partial membrane loss.
Laboratory diagnosis of ABO incompatibility is confirmed by demonstrating the appropriate “ABO set-up” (i.e., type O mother and type A or B baby) and a positive antiglobulin (Coombs) test. In the direct antiglobulin test, IgG antibody on the infant’s washed RBCs can be demonstrated. Often, this direct Coombs test is only weakly positive. Anti-A or anti-B alloantibodies often can be eluted from the infant’s cells. The indirect antiglobulin test assesses the presence of free anti-A or anti-B alloantibodies in the baby’s serum. Such a test is positive in nearly all infants with clinically significant A-O or B-O incompatibility. In general, babies whose jaundice requires phototherapy or exchange transfusion have a stronger antiglobulin reaction and more microspherocytes.
Most babies with ABO incompatibility require no therapy except that directed to the hyperbilirubinemia. Sometimes, symptomatic anemia does not manifest until 4 to 6 weeks after birth.
Rh Incompatibility
Until the late 1960s, the most frequent and severe form of hemolytic anemia in the newborn period was incompatibility between the mother and child in the major antigen of the rhesus or Rh complex (called the D antigen), resulting in the syndrome of erythroblastosis fetalis. Unlike ABO incompatibility, in which the offending maternal antibodies are natural (i.e., do not result from sensitization of the mother), Rh-negative women who develop anti-D antibody have been sensitized (immunized) either by a prior blood transfusion of D-positive blood or from that of a previous D-positive fetus. During subsequent pregnancies, anti-D antibody increases in titer, crosses the placenta, coats the fetal D-positive RBCs, and results in severe hemolysis, owing to destruction of these cells in the reticuloendothelial system. Severely anemic infants may die in utero or may be born with the syndrome of hydrops fetalis, characterized by anasarca resulting from hypoalbuminemia and congestive heart failure, severe anemia, and massive hepatosplenomegaly (resulting from cardiac failure and extramedullary erythropoiesis). The mortality rate is extremely high. Whether hydropic or not, a large percentage of these Rh-sensitized babies develop extreme jaundice, requiring multiple exchange transfusions.
The problem of Rh hemolytic disease has become less pronounced during the last three decades because of several key
research advances. First, anti-D immune globulin now is administered routinely to all Rh-negative women at 28 weeks’ gestation and immediately after delivery or after abortion; therefore, few women are sensitized. Those women who do exhibit rising titers of anti-D antibody can be identified early in the pregnancy and can be monitored by amniocentesis, serial amniotic fluid optical density measurements, and estimation of the relative risk of fetal death. Intrauterine transfusions of Rh-negative packed RBCs can be administered to correct the anemia, and planned early induction of labor—followed by vigorous management of the jaundiced, anemic, and often premature infant—has resulted in lower morbidity and mortality rates. Typically, affected babies have a strongly positive direct antiglobulin test; the indirect test usually shows the presence of large amounts of free anti-D antibodies in the baby’s and mother’s serum. The degree of anemia is variable, but usually hyperbilirubinemia is present. An elevation of the direct fraction of bilirubin is seen in the most severely affected infants, probably resulting from intrahepatic cholestasis (inspissated bile syndrome). The peripheral blood smear shows polychromasia and nucleated RBCs (erythroblastosis) but no microspherocytes, in contrast to ABO incompatibility. Treatment consists of exchange transfusion for marked hyperbilirubinemia or anemia, simple transfusions of Rh-negative packed RBCs for less severe degrees of anemia, and careful follow-up during the first 2 or 3 months of life, when delayed anemia resulting from persisting anti-D antibody or bone marrow suppression may necessitate additional transfusions.
research advances. First, anti-D immune globulin now is administered routinely to all Rh-negative women at 28 weeks’ gestation and immediately after delivery or after abortion; therefore, few women are sensitized. Those women who do exhibit rising titers of anti-D antibody can be identified early in the pregnancy and can be monitored by amniocentesis, serial amniotic fluid optical density measurements, and estimation of the relative risk of fetal death. Intrauterine transfusions of Rh-negative packed RBCs can be administered to correct the anemia, and planned early induction of labor—followed by vigorous management of the jaundiced, anemic, and often premature infant—has resulted in lower morbidity and mortality rates. Typically, affected babies have a strongly positive direct antiglobulin test; the indirect test usually shows the presence of large amounts of free anti-D antibodies in the baby’s and mother’s serum. The degree of anemia is variable, but usually hyperbilirubinemia is present. An elevation of the direct fraction of bilirubin is seen in the most severely affected infants, probably resulting from intrahepatic cholestasis (inspissated bile syndrome). The peripheral blood smear shows polychromasia and nucleated RBCs (erythroblastosis) but no microspherocytes, in contrast to ABO incompatibility. Treatment consists of exchange transfusion for marked hyperbilirubinemia or anemia, simple transfusions of Rh-negative packed RBCs for less severe degrees of anemia, and careful follow-up during the first 2 or 3 months of life, when delayed anemia resulting from persisting anti-D antibody or bone marrow suppression may necessitate additional transfusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree