Pinkal Desai, MD, MPH
Adriana Rossi, MD
Adrienne A. Phillips, MD, MPH
ACUTE MYELOID LEUKEMIA IN PREGNANCY
• Chemotherapeutic Agents Used in AML
• Treatment of AML in Pregnancy
ACUTE PROMYELOCYTIC LEUKEMIA IN PREGNANCY
ACUTE LYMPHOBLASTIC LEUKEMIA IN PREGNANCY
CHRONIC MYELOID LEUKEMIA (CML) IN PREGNANCY
LONG-TERM EFFECTS OF IN UTERO/EARLY EXPOSURE TO CHEMOTHERAPY
HEMATOPOIETIC STEM CELL TRANSPLANTATION
MECHANISMS OF GONADAL DYSFUNCTION
GRAFT VERSUS HOST DISEASE ISSUES
ASSISTIVE STRATEGIES/FERTILITY PRESERVATION TECHNIQUES
LEUKEMIAS
The spectrum of leukemia in humans can range from acute to chronic leukemia. Although acute leukemia is a hematological emergency and needs treatment urgently, chronic myeloid leukemia is a lifelong illness with relatively safe targeted therapies requiring indefinite treatment. Hematopoietic stem cell transplantation (HSCT) is an important component of treatment for acute leukemia and the only chance for long-term cure for a certain subset of patients and certainly for all relapsed patients. Leukemia in pregnancy is challenging in terms of the need for urgent therapy while trying to preserve fetal viability and the complexities of the change in pharmacokinetics and pharmacodynamics of chemotherapy in the pregnant state. Although fertility preservation is frequently not feasible due to urgency of disease state, there is potential for planned fertility preservation techniques after attaining a cure. In this chapter, we discuss the unique challenges and consequences of treatment of leukemia and effect on fertility and various fertility preservation options before and after HSCT.
ACUTE MYELOID LEUKEMIA IN PREGNANCY
Acute myeloid leukemia (AML) is a relatively uncommon malignancy with an estimated incidence of 13 per 100,000 men and women per year according to CDC data.1 The median age at diagnosis is 68 years; therefore, the incidence in pregnancy is rare with an approximate incidence of 1 in 100,000 pregnancies.2,3 However, the challenges in managing AML are significant as the disease is always aggressive by nature and delaying treatment is not an option.4 The effects on the embryo and fetus are also challenging and one has to take into account the long-term developmental effects on children after exposure to AML-directed therapy for the mother during pregnancy.
The teratogenicity of chemotherapeutic agents is usually confined to the first trimester of pregnancy. The first 2 weeks of development are usually associated with cell division and embryo expansion. Exposure to chemotherapy in this period usually leads to spontaneous abortion or no damage at all.5,6 Organogenesis occurs between 3 and 8 weeks with the most teratogenicity seen in this period. Between the weeks 8 and 38, there is growth of gastrointestinal, neural, and hematopoietic tissues. Exposure to chemotherapy in the second and third trimesters usually has potential for intrauterine growth retardation and preterm labor.5–8 Cytotoxic chemotherapy with DNA-active drugs, like cyclophosphamide and anthracyclines, has been associated with small placental size.9
Chemotherapeutic Agents Used in AML
In addition to the short- and long-term effects of chemotherapeutic agents for the fetus and mother, pregnancy poses challenges in the pharmacokinetics of these drugs. Pregnancy is associated with plasma volume expansion, increased renal and hepatic clearance of drugs, and amniotic fluid third spacing. Most chemotherapeutic agents used in the treatment of AML are of low molecular weight and can easily cross the placental barrier.5,6
Anthracyclines and cytarabine are the major drugs used in the induction and/or consolidation therapy of AML. Within the anthracycline category, idarubicin (IDA) or daunorubicin (DNR) are the two agents that are used along with cytarabine in induction of AML. IDA is more lipophilic and has a higher propensity for placental transfer and thus more potential for fetal toxicity.10,11 Therefore, DNR is the preferred anthracycline during pregnancy. Detailed anthracycline exposure has been discussed in the chapter on “breast cancer in pregnancy.”
Cytarabine exposure in first trimester has been reported to cause limb and otic abnormalities when used either alone or in combination with other agents, like 6 thioguanine, DNR, and vincristine.12–14 Other reports of transient cytopenias, intrauterine growth retardation, and rarely fetal death have also been reported.4,10 It is to be noted that most of the cases have been treated with multiagent chemotherapy protocols and individual isolated effect of a particular drug is hard to discern.
Treatment of AML in Pregnancy
AML is a hematologic emergency and delaying treatment in the mother may lead to the demise of the mother and the baby.4 Induction treatment with anthracyclines and cytarabine is the cornerstone of treatment of AML.15,16 Both categories of drugs are teratogenic in the first trimester; therefore, therapeutic abortion should be sensitively recommended before initiating treatment. If the patient refuses therapeutic abortion, DNR should be the drug of choice over IDA along with cytarabine with close fetal monitoring for potential teratogenicity. Chemotherapy during the first trimester can lead to fetal comorbidities and death in 10% to 20% of exposed fetuses.17
Induction chemotherapy in the second and third trimesters is relatively safe in terms of teratogenicity and can be used with the intent of carrying the pregnancy to term. In rare cases, if labor is imminent, chemotherapy can be delayed a few days. The timing of chemotherapy in the third trimester should be calculated whenever possible so that nadir cellular blood counts (2-3 weeks after chemotherapy) do not occur at the time of expected delivery.
Maternal and Fetal Outcomes
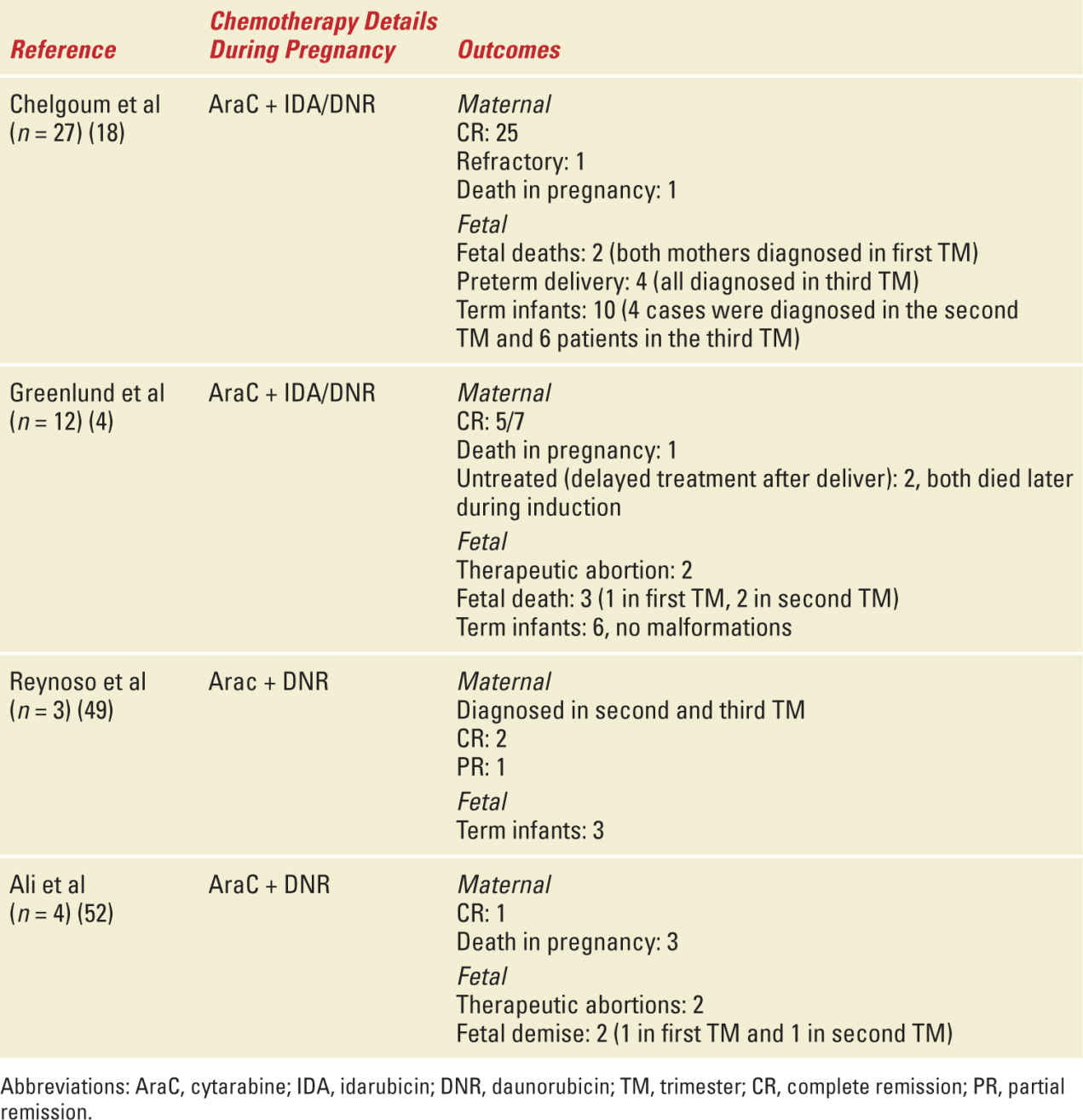
Maternal outcomes in pregnancy are not thought to be very different from nonpregnant counterparts. In a retrospective study by Chelghoum et al of 34 acute leukemia patients (including AML, APL, and ALL), 92% achieved complete remission (CR) and the overall survival rate was 64% and at 46% at 3 and 5 years, respectively.18 Cumulative data from published literature suggests a CR rate with cytarabine and anthracycline combination of 100%, 81%, and 67% in the first, second, and third trimesters, respectively.19 The estimated fetal demise/malformation rate with the use of cytarabine and DNR is 8.5% and 6.4%, respectively; for the cytarabine and IDA, combination is 28.6% and 12.5%, respectively.19 In a retrospective review of 89 cases of AML treated during pregnancy, there were 20 cases of fetal exposure to chemotherapy in the first trimester and 54 during the second and third trimesters. When chemotherapy exposure occurred in the first trimester, 3 patients had congenital abnormalities, 16 had normal term deliveries, and 1 had undergone a therapeutic abortion. In the second- and third-trimester exposures, 46 had normal term births (6 had intrauterine growth retardation), 8 had fetal demises, and 1 had a therapeutic abortion.5 Table 29-1 gives a summary of the maternal and fetal side effects that have been described.
Select Studies with Maternal and Fetal Outcomes of AML in Pregnancy |
ACUTE PROMYELOCYTIC LEUKEMIA IN PREGNANCY
Acute promyelocytic leukemia (APL) is a rare form of AML (AML M3) that constitutes 10% of AML cases and is associated with excellent long-term cure rates of more than 90% to 95%.20 However, it is commonly associated with coagulopathy and the risk of bleeding is high early in the course of presentation and treatment. This in combination with the fact that APL occurs relatively commonly in younger reproductive age patients makes it challenging to treat in pregnancy. Front line treatment of low-risk APL includes all-trans retinoic acid (ATRA) and Arsenic trioxide.21 DNR is very active in APL and is used in the presence of high-risk features, such as leukocytosis.
ATRA is contraindicated in the first trimester as its use is associated with cardiac malformations and CNS defects in 20% of babies exposed during the first trimester.21 Its use in the second and third trimesters is relatively safe and fetal malformations have not been reported. The European Leukemia Net advises against the use of ATRA in the first trimester.22 ATRA is also contraindicated during the breastfeeding.
Arsenic trioxide is contraindicated in pregnancy.22–24 In animal models, arsenic exposure is linked to fetal death, malformations, and fetal growth restriction.23–25
Because ATRA cannot be used in the first trimester, therapeutic abortion should be discussed with the patient and coagulopathy should be reversed with coagulation factor supplementation (cryoprecipitate and fresh frozen plasma) before performing the procedure. If the patient opts to continue the pregnancy, DNR can be used as a single agent and then ATRA can be added as pregnancy proceeds to the second trimester. However, single agent DNR can be associated with high risk of bleeding and close monitoring and supportive care is advised. Leukapheresis is not recommended due to a high risk of bleeding secondary to coagulopathy.
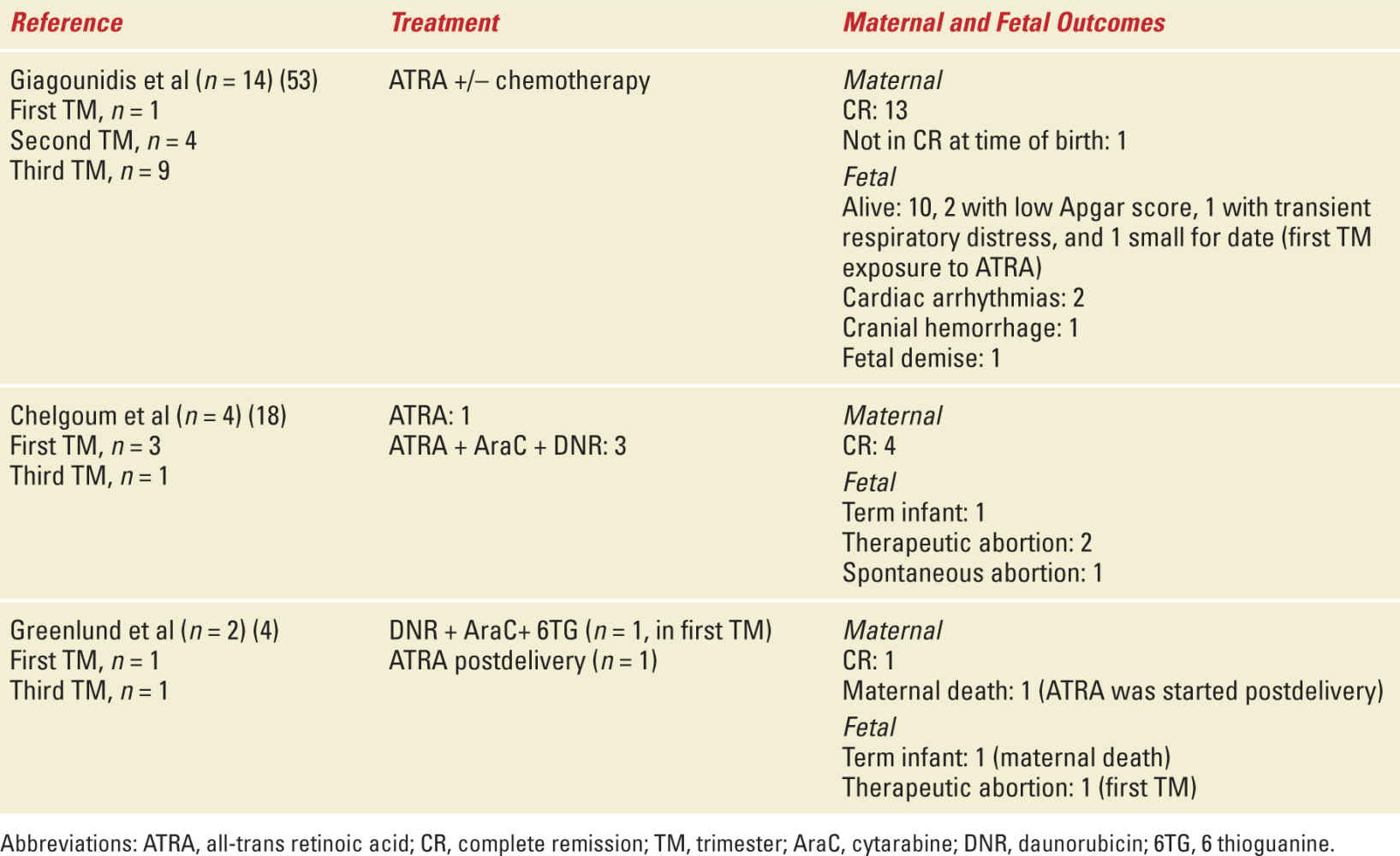
In the second and third trimester, ATRA can be used with relative safety but close fetal cardiac monitoring is recommended.26,27 ATRA can be used as a single agent but can be associated with ATRA differentiation syndrome (particularly in patients presenting with leukocytosis) and ATRA resistance.28 Therefore, a combination of ATRA and DNR is optimal in the second and third trimester. In some cases when delivery is imminent and being induced, ATRA can be used as a single agent with DNR added postdelivery. Induced vaginal delivery at term (or at 32 weeks with corticosteroid support) is preferable to caesarean section due to the risk of bleeding and DIC associated with APL. Table 29-2 shows select studies of maternal and fetal outcome in APL with pregnancy.
Select Studies with Maternal and Fetal Outcomes of APL in Pregnancy |
ACUTE LYMPHOBLASTIC LEUKEMIA IN PREGNANCY
Acute lymphoblastic leukemia (ALL) is mostly seen in children and adolescents and constitutes a relatively uncommon leukemia in adults compared with AML. Therefore, data on treatment of ALL in pregnancy is limited. The treatment of ALL is long, comprising of induction, consolidation, and maintenance along with intrathecal chemotherapy and sometimes CNS radiation. The duration of treatment extends to 2 to 3 years after the initial intensive chemotherapy.
There are many drugs that are active in ALL including cytoxan, DNR, methotrexate, mercaptopurine, thioguanine, asparaginase, glucocorticosteroids, and vincristine. A multiagent chemotherapy regimen is usually used in the induction and consolidation phases while POMP (prednisone, vincristine, methotrexate, and mercaptopurine) is the mainstay in the maintenance phase. Many drugs used in treatment of ALL (vincristine and 6MP) have trophoblastic growth inhibition properties in the human placenta while methotrexate has severe trophoblastic toxicity.29 High-dose methotrexate in the first trimester is associated with multiple fetal malformations including cleft palate and the very well-described aminopterin syndrome (cranial dysostosis, micrognathia, hypertelorism, and external ear abnormalities).30,31 Low-dose methotrexate may not be as teratogenic in the first trimester but it is of limited value in the induction phase.32 Second- and third-trimester exposures to methotrexate have not been associated with significant malformations. Asparaginase is known to cause pancreatitis, liver function abnormalities, and thrombosis. Although it has been used in pregnancy in some cases, close monitoring is required due to the hypercoagulable state observed in pregnancy.
As there is less data on the outcomes of pregnancy with ALL diagnosed in the first trimester, therapeutic abortion should be discussed. If ALL is diagnosed in the late first trimester, a short-term glucocorticosteroid course can be used to allow transition to the second trimester when chemotherapy may be started. In the second and third trimesters, induction strategies with multiple agents excluding methotrexate are usually recommended. In a review of 60 reported outcomes of pregnancies in the literature, there were 38 cases of first trimester and 22 cases of second- and third-trimester exposures to multiagent chemotherapy. In cases of first trimester exposure, no abnormalities were observed in 32 cases, while congenital abnormalities (n = 2), spontaneous abortion (n = 1), maternal death (n = 1), and IUGR (n = 3) were observed in the rest. In cases of second- and third-trimester exposures, normal development was observed in 17 cases, while fetal cytopenias (n = 2), fetal death in utero (n = 1), stillbirth (n = 1), and transient cardiomyopathy (n = 1) were observed in the other cases.33
CHRONIC MYELOID LEUKEMIA (CML) IN PREGNANCY
Chronic myeloid leukemia (CML) affects 1 in 100,000 pregnancies and is unique in this setting as the disease is associated with an elevated thrombotic risk secondary to the myeloproliferative nature of CML coupled with the hypercoagulable state of pregnancy.33 Patients usually need to be treated with tyrosine kinase inhibitors (TKIs) for the rest of their lives and thus present with unique scenarios of not only unplanned but also planned pregnancies while on this lifelong treatment.
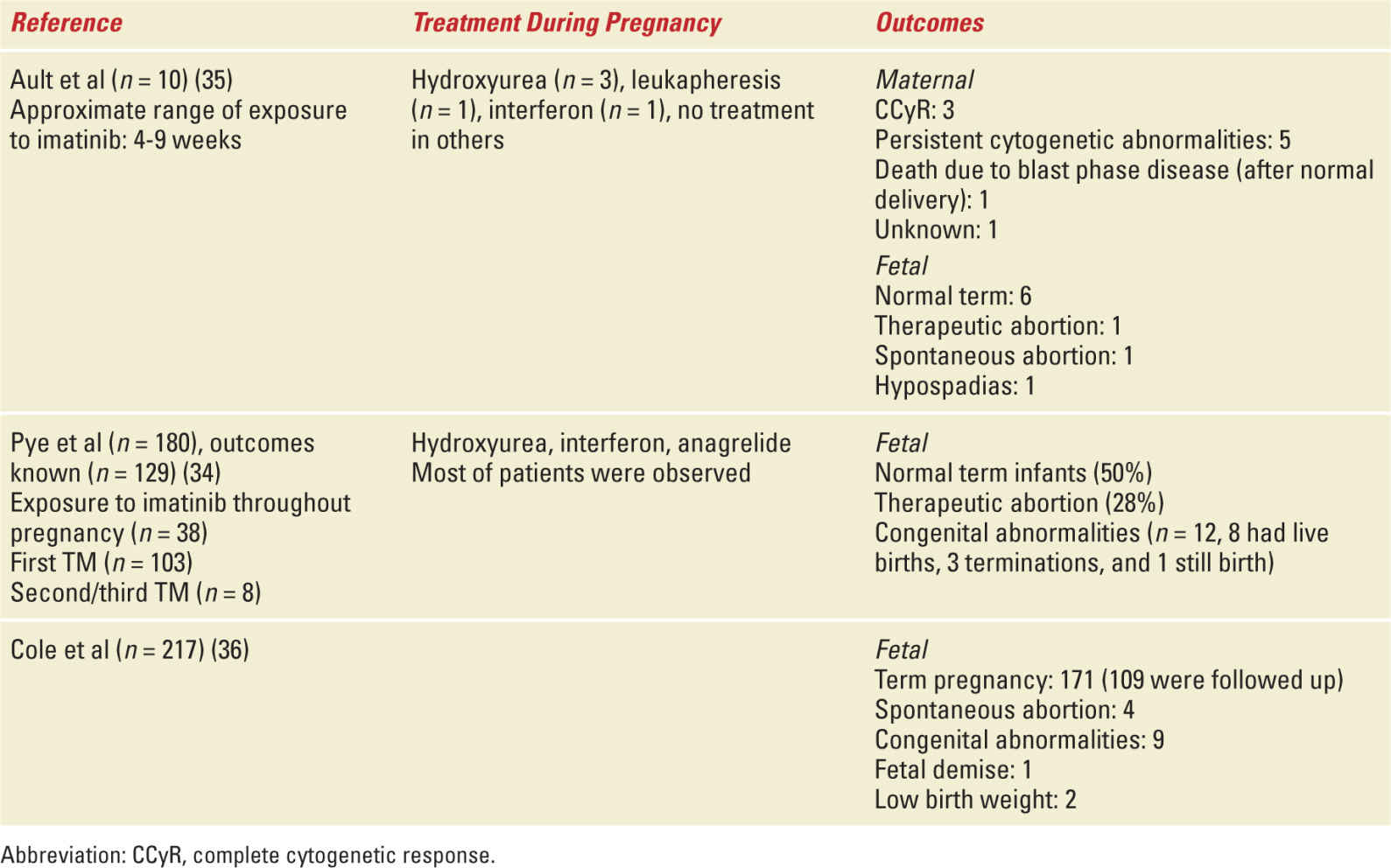
Imatinib is the TKI that has the most data on pregnancy and is considered a teratogen. Its use is contraindicated during organogenesis and it has been associated with multiple fetal malformations including skeletal anomalies, duplex kidney, renal agenesis, hypoplastic lungs, hypospadias, and omphalocele.34,35 There is no extensive safety data on the use of imatinib during the second and third trimesters and it should be avoided. However, there are case reports of successful use of imatinib in the second and third trimesters without adverse outcomes.36 Second generation TKIs (dasatinib and nilotinib) can cross the placenta.37,38 Dasatinib and nilotinib have minimal data in terms of teratogenicity and pregnancy outcomes after exposure. There have been isolated reports of both nilotinib and dasatinib that suggest no adverse outcomes after limited exposure during the first trimester.39,40 One report of dasatinib exposure was associated with fetal bicytopenias, pleural effusion, and hydramnios when exposed during organogenesis.37
Interferon is considered safe in the second and third trimester, and does not cross the placental barrier. It is the drug of choice in treatment of chronic phase CML in pregnancy.41
Chronic phase CML in the first trimester can be treated with observation with close attention to the prothrombotic state. Low molecular weight heparin can be used when platelet counts exceed 1 million. Leukocytosis (WBC >100,000/micro L) can be controlled with leukapheresis as tolerated and then interferon can be initiated in the second trimester. All TKIs should be avoided during pregnancy. CML in blast crises should be treated with the multiagent chemotherapy employed in acute leukemia.
Pregnancy can be planned in patients who are controlled on TKIs. There have been recent data suggesting that if patients are in complete molecular response (CMR), stopping imatinib can be an option and 40% of patients continue to be in CMR after stopping the agent.42 Patients should be advised to wait ideally until sustained CMR is reached before pursuing pregnancy. However, not many patients reach CMR and in that case, a sustained major molecular response (MMR) is acceptable. Second-generation TKIs (dasatinib and nilotinib) reach MMR faster and may be good agents to switch to if patients are taking imatinib with suboptimal responses.43,44 Once a favorable response is achieved, TKIs should be stopped before ovulation and patients closely followed with Reverse transcriptase Quantitative Polymerase Chain Reaction every 2 months. Interferon can be initiated if there are major concerns on loss of response.
TKIs should be reinitiated postpartum. All TKIs are secreted in breast milk, and breastfeeding is contraindicated while patients are receiving this treatment. Table 29-3 depicts outcomes of pregnancies complicated with CML.
Select Studies with Maternal and Fetal Outcomes of CML in Pregnancy |
LONG-TERM EFFECTS OF IN UTERO/EARLY EXPOSURE TO CHEMOTHERAPY
There have been a few studies that have demonstrated no increase in risk of growth retardation or neurologic decline in children who have been exposed to chemotherapy in utero.45–49 Although most of these studies had patients exposed to chemotherapy for nonleukemic cancers, no particular trend was noted for patients exposed to acute leukemia-directed therapies.
ALL is common in children and adolescents and long-term fertility and effect on future offspring is a potential concern when chemotherapy exposure occurs during gonadal development. One study of more than 2000 childhood ALL patients exposed to chemotherapy treatment observed no increased abnormalities or cancer risk in their offspring.50
Long-term fertility has been studied in children exposed to leukemia-directed chemotherapy and gonadal dysfunction is correlated with the cumulative chemotherapy and cranial radiation exposure.51–53
Avoiding pregnancy while undergoing chemotherapy is essential and all childbearing age women should have contraceptive measures in place. Of particular note is that amenorrhea is not uncommon during chemotherapy and it does not mean fertility is definitely impaired during this time. Many patients who are undergoing maintenance with POMP for ALL can have a return of menstruation while on POMP and should continue to be on contraceptive measures. Fertility preservation should be discussed before initiating leukemia treatment but frequently this is not practical due to the need for emergent treatment of acute leukemia.
LYMPHOMAS AND MYELOMAS
Lymphoma and myeloma are malignancies of mature lymphocytes. Multiple myeloma (MM) is characterized by an excess of plasma cells in the marrow with aberrant production of monoclonal antibodies. It is usually diagnosed in the seventh decade of life, and as such it is rarely seen in pregnancy. In contrast, lymphoma generally involves secondary hematopoietic organs and is the fourth most common malignancy diagnosed during pregnancy. There are many subtypes of lymphoma, characterized by the cell of origin, which vary greatly in pathology and clinical presentation. The primary distinction is between Hodgkin Lymphoma (HL) from Non-Hodgkin lymphomas (NHL). Diagnosis is often delayed due to nonspecific clinical presentation—such as fatigue, shortness of breath, and weakness—and reluctance to pursue diagnostic testing in the pregnant patient.54
Lymphoma
Hodgkin Lymphoma
CLINICAL PRESENTATION—HL has a bimodal age distribution, the first being coincident with child-bearing age. It is the most commonly seen hematologic malignancy in pregnancy, classically presenting with enlarging lymphadenopathy of the mediastinum, chest, and/or neck, resulting in fatigue and shortness of breath.55
DIAGNOSIS STARTS—With detailed patient history with particular attention to B-symptoms (fever, night sweats, weight loss) and directed physical examination focused on palpable lymph nodes. Laboratory evaluation should include a complete blood count, chemistries, and liver function evaluation, as well as lactate dehydrogenase and ESR. Positron emission tomography/computed tomography (PET/CT) scanning is the gold standard imaging modality in HL; however, it should be avoided during pregnancy given the high dose of ionizing radiation and placental exposure of radiolabeled isotopes.56 Ultrasonography may be used for abdominal evaluation, in combination with chest x-ray using abdominal shielding. MRI without contrast has been found to be safe if necessary. Final diagnosis requires excisional or core biopsy of the involved lymph node for evaluation of Reed-Sternberg cells, and bone marrow biopsy is used for staging. These are usually minimally invasive procedures, except in rare cases where access to the lymph node proves challenging.57
MANAGEMENT—Therapeutic options must be discussed in a multidisciplinary team, involving hematology, high-risk obstetrics, and neonatology as well as the patient and family. Gestational age and stage of disease will help dictate therapy. Risk of fetal toxicity depends on type and dose of drug, as well as timing and duration of exposure.58 HL and pregnancy have not been shown to adversely affect each other, and pregnancy termination has not been shown to improve outcomes. Early-stage disease can usually be monitored until delivery, but symptomatic patients may require emergent treatment.59 There are a number of case reports and case series as well as retrospective studies, but no prospective data is available for treatment of HL in pregnancy.
In the largest retrospective study of nearly 8 million births, 600 patients with HL diagnosis at time of birth admission were identified. These patients demonstrated increased incidence of venous thromboembolic events and greater need for packed red blood cells (PRBC) transfusion, and higher rates of preterm labor was noted. However, there were no differences in the many other complications of pregnancy evaluated, and no increase in maternal or fetal mortality.60
Even in advanced stage disease, HL is a highly curable disease. The standard of care is combination therapy with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine). Toxicities include fatigue, neutropenia, nausea, and alopecia, which can be safely managed in pregnancy. Response and survival outcomes in the pregnant patient are similar to nonpregnant counterparts when treated in a timely manner.61
ABVD has been used during all three trimesters and is generally thought to be safe in the second and third trimesters, with rare fetal adverse effects, even with long follow-up. Low birth weight has been reported, but no neurologic difficulties or difference in school performance were noted, and there was no increase in secondary malignancies.62 There are conflicting reports of use during first trimester, with increased risk of spontaneous abortions and fetal complications reported.63 Therapy should be avoided within 2 to 3 weeks of delivery, and may be deferred if diagnosis is made late in the third trimester. In order to minimize maternal and potential fetal toxicity of bleomycin and dacarbazine, the use of EVA (etoposide, vinblastine, doxorubicin) during pregnancy followed by ABVD upon delivery has been used with success.64
The use of radiotherapy in HL is reserved for limited and asymptomatic disease, or in combination with chemotherapy. Due to exposures exceeding the recommended 0.1 Gy to the fetus, it ought to be deferred until after delivery. Although PET/CT scans should be avoided during pregnancy, patients should have posttreatment PET/CT to evaluate response.65
Non-Hodgkin Lymphomas
There are a number of lymphoma subtypes, which differ greatly in pathophysiology and clinical course. Most are B-cell malignancies, with T-cell comprising approximately 20% of cases. They are further divided into indolent and aggressive subtypes. Although the former can usually be monitored without intervention, aggressive lymphomas can be rapidly life threatening and require timely diagnosis and treatment. Indolent lymphomas diagnosed in pregnancy can usually defer any therapy until delivery. The most common aggressive B-cell lymphomas are diffuse large b-cell lymphoma (DLBCL) and Burkitt lymphoma (BL).
CLINICAL PRESENTATION—DLBCL is the most common NHL subtype and can occur at any age. Patients usually present with B-symptoms and rapidly progressive lymphadenopathy or extranodal disease, such as primary mediastinal or CNS lymphomas. Presentation with fatigue, malaise, and shortness of breath are most common, and often confused for normal pregnancy.63
BL is most common in children, but does comprise the second most common NHL in pregnancy. It is the most aggressive subtype with very high proliferation rates, and requires immediate combination therapy to prevent maternal demise. Interestingly, involvement of reproductive organs is more common in the pregnant patient and breast involvement is often bilateral.66,67
DIAGNOSIS—Similar to HL, evaluation should start with pointed history and physical examination. Laboratory evaluation should include complete blood count, renal and hepatic function evaluation, and lactate dehydrogenase with further evaluation of viral serologies as indicated. Imaging with chest x-ray and ultrasonography are safe, but evaluation may require further investigation. MRI has been shown to be safe, and in life-threatening situations, use of CT scans has been reported. Tissue biopsy is essential, including core or excisional biopsy of the affected lymph node or mass as well as the bone marrow.68
MANAGEMENT—Therapies for lymphoma usually require combinations of multiple chemotherapeutic agents, and may be curative especially in early stage disease.
Standard therapy for DLBCL is R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Its use has been described in all three trimesters without increased toxicity to the fetus. It is preferably given in second and third trimesters, where the only complications often seen are low birth weight and transient fetal B-cell depletion. Use of anthracyclines should be avoided during the first trimester.69,70
The use of rituximab, a monoclonal antibody targeting the B-cell marker CD20 has become standard of care for most lymphomas expressing this target.71 It is an IgG1 molecule and can cross the placenta, increasingly so up to 26 weeks gestation when the concentration in the fetal circulation reaches maternal level. Although maternal exposure may lead to B-cell depletion in the fetus for months after infusion, it is self-limited, with only 1% infections reported, and no increase in congenital abnormalities.72 Unintentional use of rituximab during the first trimester has been reported with no increase in ill effects.73,74
Therapy for primary CNS lymphoma usually requires high dose methotrexate (MTX) in order to overcome blood brain barrier. This drug is contraindicated in pregnancy, and has been shown to cross the placenta even when given intrathecally, possibly due to increased permeability of the blood brain barrier in pregnancy. Use of single agent rituximab during pregnancy given as 4 weekly doses in a patient with rapidly progressive neurologic deficits due to primary CNS lymphoma has been reported with rapid resolution of symptoms. The infant was noted to have depletion of B-cells and required IVIG therapy, but was fully recovered by age 4 months. The patient went on to complete standard therapy after delivery.75
Diagnosis of aggressive lymphoma in the first trimester is particularly difficult, and termination should be considered. Attempts to delay treatment until the second trimester may compromise maternal survival. Stefos et al reported a case of a very aggressive lymphoma presenting with a rapidly growing neck mass at 10-week gestation, leading to respiratory compromise in the following weeks. This patient was successfully treated with 8 cycles of CEOP (cyclophosphamide, etoposide, vincristine, and prednisolone) without ill effects. Within 3 weeks, the woman had no evidence of disease.76
Burkitt lymphoma is very aggressive, and rapidly fatal given its high proliferation index. Standard therapy is complex and includes rituximab, MTX, doxorubicin, cyclophosphamide, vincristine, ifosfamide, cytarabine, etoposide, and intrathecal MTX/Arac. Given the high mortality rate and availability of potentially curative therapy, multiagent treatment should be started as soon as possible, with clear understanding of toxicities and participation of a multidisciplinary team.77
Cases of BL in pregnancy have been treated with multiple regimens. CALGB 10002 regimen has been used during the second and third trimester with delivery of a healthy baby. Use of hyper-CVAD and the McGrath protocol without the intrathecal components have been reported with cure of the patient, but resulting fetal loss. Both of these did not impact future fertility.77
Multiple Myeloma
CLINICAL PRESENTATION—Less than 4% of patients are diagnosed with myeloma are under the age of 40.78 Fatigue is the most common symptom. Most patients present with anemia, renal insufficiency or bone pain.
DIAGNOSIS—Focused history and physical examination followed by laboratory evaluation. Evaluation consists of blood test for disease burden (serum protein electrophoresis with immune-fixation, LDH, beta-2-microglobulin) and evidence of end organ damage (CBC, CMP, BNP, troponin). Blood and urine immunofixation should be pursued. Bone marrow biopsy or biopsy of suspected plasmacytoma is required. PET/CT is the best imaging, but may be deferred if there is other evidence of end-organ damage.
MANAGEMENT—Evidence of end-organ damage dictates the need for therapy. Those without bone or renal impairment may be monitored through the pregnancy, while symptomatic patients should be started on therapy. Dexamethasone is safe as single agent, and may lead to sufficient disease control until time of delivery. Derivatives of thalidomide should be avoided during pregnancy. There is limited data for use of bortezomib in pregnancy; however, preclinical studies do not support its use and the FDA has given it a category D. Patients with bone disease should receive bisphosphonate therapy in order to prevent skeletal related events.79 Bisphosphonates are usually contraindicated during pregnancy due to risks of fetal/neonatal skeletal anomalies and hypocalcemia. If absolutely required, extensive counseling to the patient must be undertaken. Case reports in the literature have reported the use of bisphosphonates in pregnancy with no serious side effects.
The most recent review identified 28 cases of myeloma in pregnancy reported in the literature. Nearly half were reported before availability of novel agents. Of the fourteen patients diagnosed during pregnancy, five received treatment and noted no ill effects on the fetuses.80 One series of six patients reports treatment with CMOP (cyclophosphamide, melphalan, vincristine, prednisone) and DAI (dexamethasone, all-trans retinoic acid, and interferon). All patients had bony involvement and delivered by cesarean section. Three patients went on to receive autologous stem cell transplantation (ASCT), two achieved improved responses but later relapsed, and one died of progressive myeloma. All newborns were normal, no congenital abnormalities were noted, and with years of follow up no physical or psychological consequences were identified.81
An Australian group reported two more cases, one diagnosed at the time of a spontaneous abortion, successfully treated and relapsing during a future pregnancy. The other was diagnosed in the first trimester with extensive bone disease and Bence-Jones proteinuria. Termination of pregnancy was sought and the patient received chemotherapy and radiation followed by ASCT. The patient achieved a complete response, and was followed during several relapses and lines of therapy for another 10 years.82
Three more cases were seen in the United Kingdom, one diagnosed in the third trimester with extensive bony disease, another had progressive Bence-Jones proteinuria in the second trimester, and the third presented with cytopenias in the first trimester. All three patients were treated with dexamethasone until delivery by c-section.83
A number of patients have been noted to have relapse of their previously diagnosed myeloma during pregnancy. Physiologic changes of pregnancy, including relative immune impairment of the immune system via regulatory T-cell expansion and altered cytokine expression, likely contribute to this phenomenon. Myeloma cells are highly dependent on interactions with the microenvironment for survival, by both direct cell-cell contact and through cytokine modulation. One such cytokine, interleukin-6, has been shown to be elevated during pregnancy.84
Pregnancy Considerations
As with any malignant diagnosis during pregnancy, evaluation and treatment of lymphoma or myeloma requires coordinated care within a multidisciplinary team. The risk to maternal survival must be weighed against the risk to the fetus. The most common diagnosis is of HL, which has been successfully treated with curative intent and minimal toxic effects during the second and third trimesters. Indolent NHL may be monitored and usually does not require therapy before delivery. Aggressive NHL usually requires immediate intervention to ensure maternal survival. Myeloma is rarely seen, and many times can be managed during pregnancy with dexamethasone, deferring more aggressive therapy after delivery. As with other malignancies, gestational age, stage and symptoms of disease, selection, and dose of therapy are all important considerations in developing a treatment plan. Physiologic changes in pregnancy result in changes in volume of distribution, metabolism, and excretion of drugs due to increase plasma volume and third space due to amniotic fluid.85 Delivery should be planned 2 to 3 weeks after completion of chemo for bone marrow recovery.
HEMATOPOIETIC STEM CELL TRANSPLANTATION
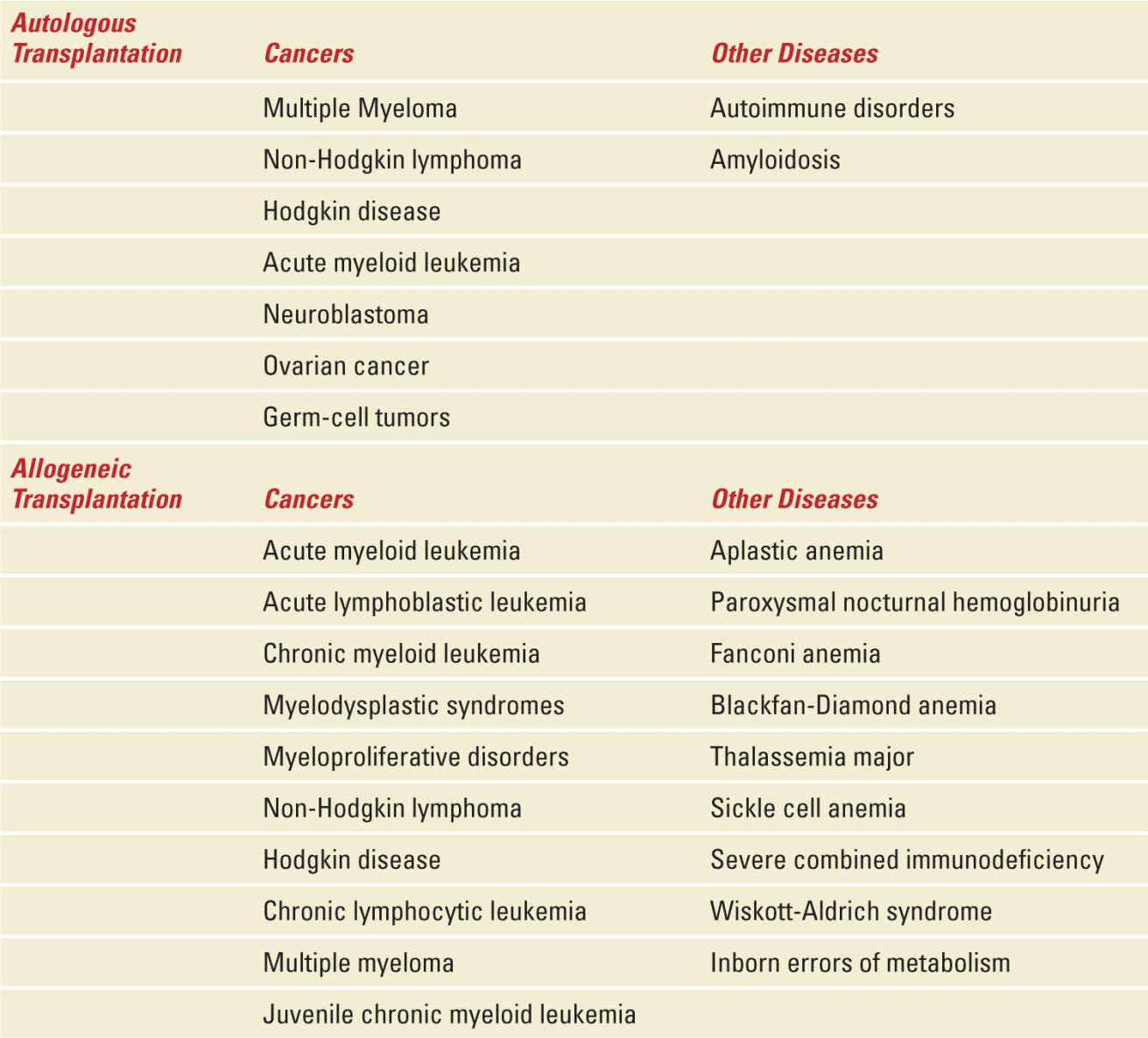
HSCT refers to a series of procedures in which a patient is treated with chemotherapy, radiation therapy, or both, followed by the administration of hematopoietic progenitor cells from any source (eg, bone marrow, peripheral blood, umbilical cord blood) or donor (eg, allogeneic or autologous). Hematopoietic stem cell transplantation (HSCT) has become a standard of care for many patients with select congenital or acquired disorders of the hematopoietic system and for patients with hematologic malignancy (Table 29-4).86 According to the Center for International Blood and Marrow Transplant Research program, approximately 20,000 HSCTs were reported in the United States in 2012.87 The widespread use of HSCT can be attributed to its improved efficacy, wider variety of stem cell sources, improved transplantation strategies, and supportive care. With broadening indications and improvement in survival, the number of long-term HSCT survivors is expected to increase steadily. The majority of HSCT are performed on recipients younger than 40 years of age, with more than 12,000 HSCT procedures per year taking place among recipients of 20 years or younger.88 Quality of survival data indicates largely normal work and marriage lifestyle among these survivors who received high-dose systemic cancer treatment; however, permanent gonadal failure and infertility are known toxicities.89 Although successful pregnancies after HSCT have been reported, evidence suggests that female survivors may be at increased risk of spontaneous abortions and miscarriages, preterm delivery and low birth weight babies. Healthcare providers who treat HSCT survivors must be informed of these long-term treatment effects and be familiar with the fertility-related treatment needs of survivors. This section will summarize the current knowledge on HSCT and its impact on maternity.
Diseases Commonly Treated with Hematopoietic Stem-Cell Transplantation |
STUDIES OF FERTILITY
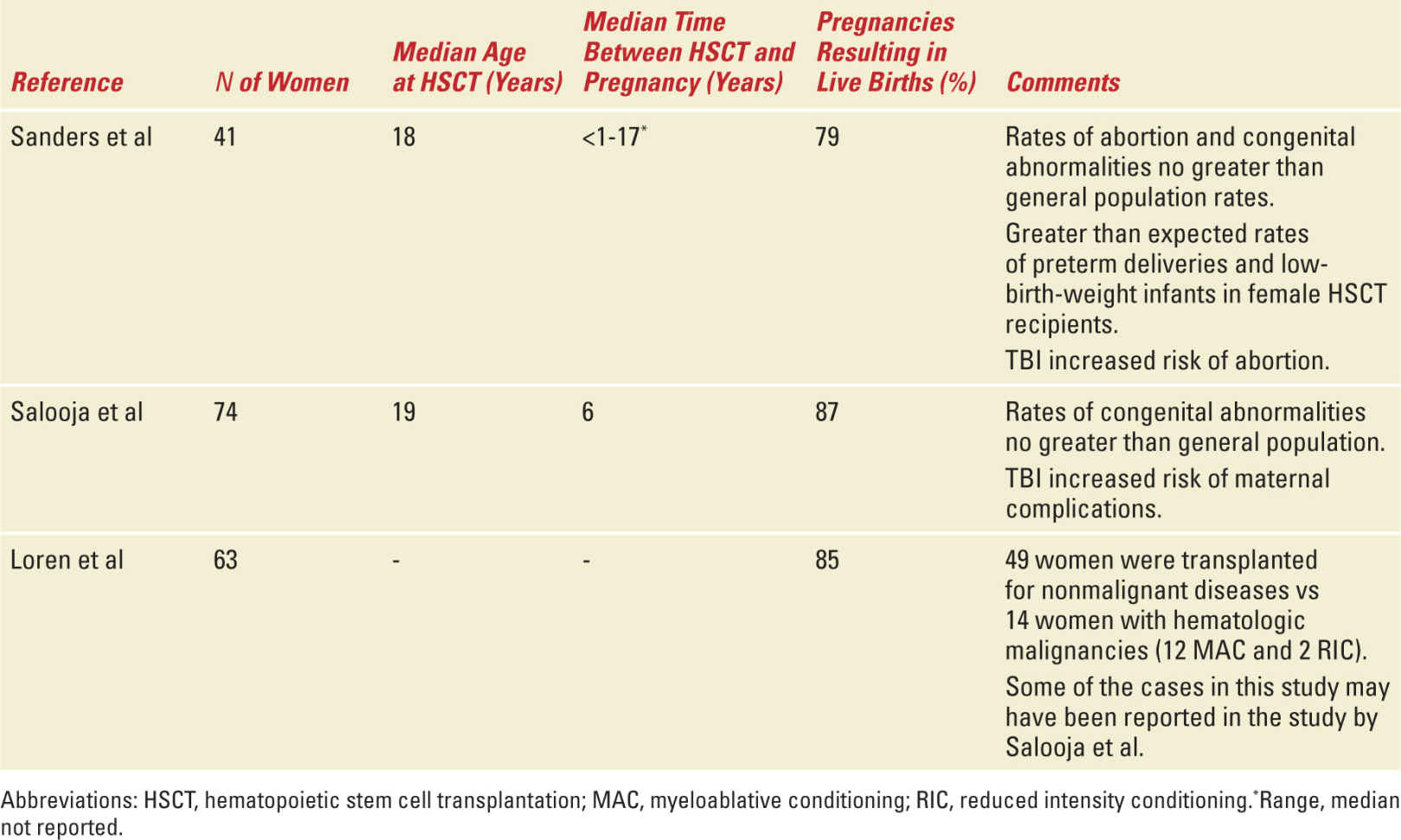
The overall prevalence of pregnancy following HSCT is low; however, the true magnitude of infertility following HSCT will always be difficult to determine because of a lack of complete data on pre- and posttransplant fertility status as well as lack of information regarding the desire to become pregnant after undergoing HSCT. Multiple case reports and case series as well as registry-based reviews have described pregnancy outcomes among autologous and allogeneic HSCT recipients (Tables 29-5 and 29-6). The reported literature suggests that pregnancies among HSCT survivors and their partners do in fact result in live births; however, as successful pregnancy outcomes are more likely to be reported than other outcomes, these limited experiences must be reviewed cautiously.
Studies of Pregnancy Outcomes After Allogeneic HSCT |
Studies of Pregnancy Outcomes After Autologous HSCT |
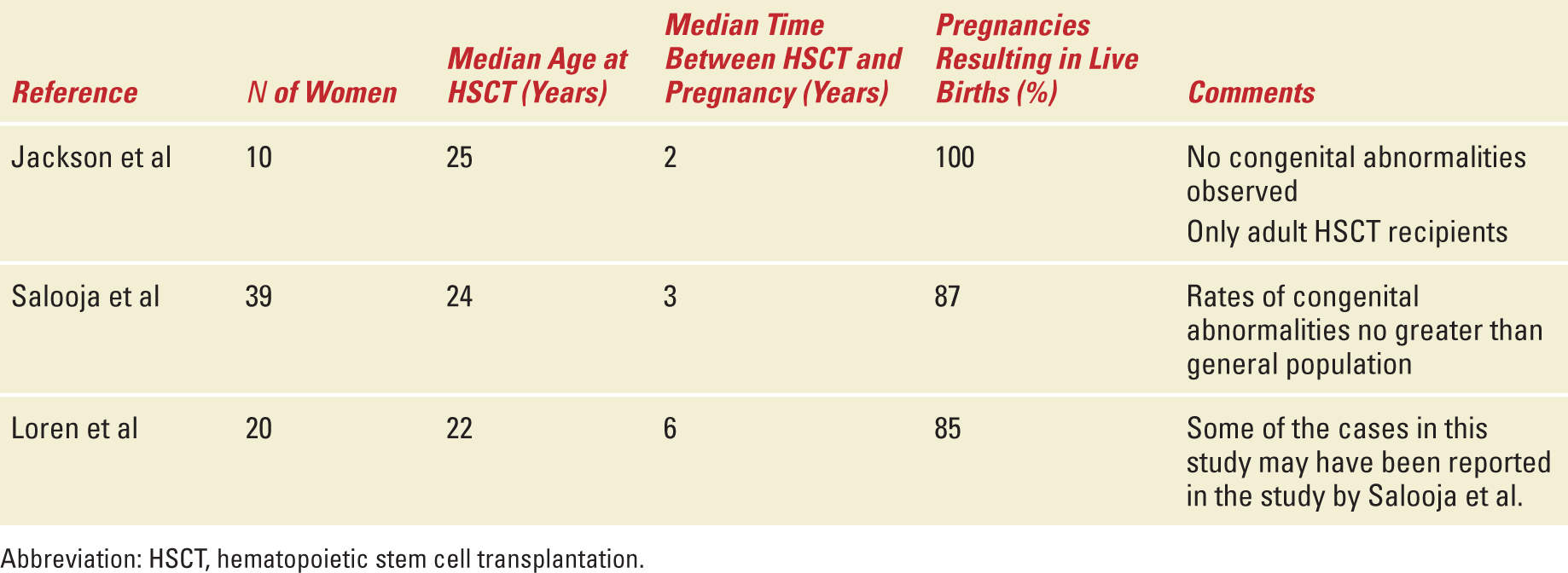
The largest US study conducted by Sanders et al reports the Seattle experience between 1971 and 1992 of the 1522 disease free survivors of allogeneic HSCT.90 This group included 708 postpubertal women of whom, 110 recovered normal ovarian function and 32 became pregnant. An additional nine previously prepubertal girls (age 3-11 years old) with normal gonadal function became pregnant. Of the total 32 postpubertal female patients with pregnancies, the median age at HSCT was 30 years; the median age at first pregnancy was 24 years and the median time between HSCT and pregnancy was 3 years. The overall frequency of spontaneous abortion in this series was comparable to the general population. The authors concluded that the data did not support that HSCT resulted in an increased spontaneous abortion rate. Pregnancies among female patients in this series were however associated with an increase incidence of preterm labor (25% compared with 8%-10% expected in the general population) and delivery of low birth weight or very low birth weight babies (25% compared with 6.5% expected in the general population). The babies born did not seem to be at an increased risk for congenital abnormalities. The increase risk of preterm and low or very low birth weight babies may be related to exposure to total body irradiation (TBI) which is thought to induce structural changes in the uterus, including reducing the elasticity of the uterine musculature and/or producing uterine vascular damage.91
In a retrospective study from the European Group for Blood and Marrow Transplantation of patients receiving both allogeneic and autologous HSCT, the frequency of pregnancy complications was higher among women who received allogeneic but not autologous HSCT.92 There was a significantly higher than normal rate of preterm delivery and low birth weight babies and this was confined to women who had received TBI in the pretransplant conditioning. Interestingly, this was also most striking in those who had conceived by artificial reproductive techniques. In addition, female HSCT survivors were at higher risk of undergoing cesarean section compared with the normal population; however, for many, a specific reason for cesarean section was not reported. This observation may be related to the perception that HSCT survivors have higher risk pregnancies that require different management than the general population.
In another study comparing the prevalence of conception and pregnancy outcomes after HSCT with a sibling comparison group from the Bone Marrow Transplant Survivor Study, the prevalence of conception following HSCT was significantly diminished compared with siblings with a comparable age and gender distribution.93 However, if pregnancy did occur, the outcome was likely to be favorable. Eighty-five percent of known pregnancies among HSCT survivors resulted in a live birth, compared with 75% of those reported by the nearest-age siblings and 62% of those reported by the Centers for Disease Control/National Center for Health Statistics on trends in the United States in Pregnancy Rates by Outcome. After adjusting for key variables, the HSCT survivors were as likely as sibling controls to report a pregnancy ending in live birth and survivors were not more likely to report a pregnancy ending in miscarriage or stillbirth.
MECHANISMS OF GONADAL DYSFUNCTION
Studies specifically assessing fertility after HSCT are limited as they do not account for whether patients are trying to conceive. However, it is estimated that ovarian failure after HSCT is observed in 65% to 84% of transplant recipients.93,94 Oocyte numbers are approximately two million at birth in the female fetus and decline thereafter. This age-dependent ovarian reserve is a determinant of the relative impact of cytotoxic therapy on overall fertility.95 In addition, granulosa cells that support oocytes within developing ovarian follicles are exquisitely sensitive to radiation, especially during the early and intermediate stages of follicular development.96 During late follicular development, granulosa cells are no longer dividing and thus are more radioresistant. Even if doses of radiotherapy or chemotherapy are insufficient to induce immediate acute ovarian failure, there may still be a reduction in the ovarian reserve rather than outright acute ovarian failure. This may manifest later as premature menopause.97
Clinical indicators of ovarian failure include failure to go through puberty, absent menstruation (primary or secondary), and symptoms of menopause. Laboratory indicators of ovarian failure include elevated serum follicle-stimulating hormone and luteinizing hormone coupled with low estradiol levels, a low level on anti-Mullerian hormone (AMH) and reduced numbers of antral follicles on ultrasound. AMH value has not been shown to guide fertility predictions in lymphoma survivors posttransplant.98 A higher ovarian volume or a higher number of antral follicles, before treatment starts may ensure a greater chance of successful pregnancies; however, research is still ongoing.
Transplant preparative regimens include various combinations of alkylating agents [cyclophosphamide (CY), busulfan, melphalan], antimetabolites (fludarabine), and irradiation [total-body irradiation (TBI), thoraco-abdominal irradiation, total lymphoid irradiation]. Exposure to these agents is associated with gonadal failure. Sanders et al reported the experience of 187 women between 13 and 49 years of age that had ovarian function evaluated from 1 to 15 years (median 4) after HSCT for aplastic anemia or leukemia.99 Among 43 women transplanted for aplastic anemia following 200 mg/kg of CY, all 27 women younger than 26 years of age, but only 5 of 16 women older than 26 years of age recovered to normal ovarian function. Nine of the 43 have had 12 pregnancies resulting in 2 normal live births, 2 elective abortions, and 2 spontaneous abortions. Nine of 144 women transplanted for leukemia following 120 mg/kg of CY and 9.20 to 15.75 Gy TBI recovered ovarian function. Two of these nine patients have had three pregnancies resulting in two spontaneous and one elective abortion. By 7 years after transplant, the probabilities of having normal ovarian function were 0.92 after CY alone and 0.24 after CY plus TBI (p<0.0001). Multivariate analysis showed that TBI was the only factor significantly influencing ovarian failure and that both TBI and greater patient age at transplant were significantly associated with a decreased probability of recovering normal ovarian function. These data demonstrate that the risk of gonadal dysfunction is higher with increasing doses of alkylating agents, TBI, and older age at time of transplant. Reduced intensity conditioning or nonmyeloablative HSCT may avoid the necessity for high doses of cytotoxic agents and TBI without compromising disease-free survival; however, this has not been studied.
GRAFT VERSUS HOST DISEASE ISSUES
Before transplantation, the hematopoietic and immunologic systems of the recipient are ablated (or nearly ablated) with supralethal doses of chemotherapy with or without radiation. Subsequent recovery of marrow function depends on successful repopulation by donor (graft) cells. A clinicopathologic syndrome known as graft-versus-host disease (GVHD) may complicate HSCT when immunologically competent donor lymphocytes react against histocompatibility antigens of the host. This can occur both acutely and chronically. Within 3 months of transplant, acute GVHD is evidenced by skin rash, hepatic dysfunction, and gut abnormalities. This reaction appears to set the stage for chronic GVHD which can manifest 3 to 12 months after hematopoietic stem cell grafting and affects approximately one third of long-term transplant survivors.100 There is limited information on GVHD and fertility; however, it is know that chronic GVHD alters elastic fibers in the skin and vaginal vault and may cause vaginal dryness, vaginal narrowing, and inflammation of the vaginal wall.101 In Sanders study, only three of nine pregnancies with preterm labor occurred in women with previous GVHD and its causal association is unknown.5 In a recent study of a mouse model of nonirradiated bone marrow transplantation, the ovary was found to be a target of GVHD and administration of prednisolone significantly reduced ovarian GVHD and restored numbers of oocytes in allogeneic animals suggesting that control of GVHD may be important to prevent female infertility after HSCT.102
ASSISTIVE STRATEGIES/FERTILITY PRESERVATION TECHNIQUES
Assisted reproductive techniques such as sperm or oocyte cryopreservation, in vitro fertilization, or pharmacological interventions play an unclear role in the rate of posttransplant pregnancy. However, careful thought should be given before transplant to possible strategies to preserve fertility. Table 29-7 highlights recommendations for fertility preservation in HSCT recipients. The American Society of Clinical Oncology 2013 guidelines recommends that all cancer patients of childbearing age be referred to a reproductive endocrinologist.103 A survey of US Oncologists, however, found that only 47% of respondents routinely followed this practice.104 Fertility preservation among HSCT patients presents several challenges. Patients frequently have diseases that are at high risk for relapse and delaying therapy to pursue fertility preservation may not be perceived as an option. Despite this, a study of transplant physician perceptions and practice patterns by Loren et al suggests that most transplant physicians were knowledgeable and comfortable discussing fertility issues relevant to their patients, particularly when compared with general oncologists. However, educational materials and collaborative relationships with colleagues who are experts in fertility preservation were not always available.105
Recommendations for Fertility Preservation in HSCT Recipients, Loren et al JCO 2013 |



