Hematologic Agents
Annie Nguyen-Vermillion
Sandra E. Juul
Introduction
The bone marrow of a healthy growing fetus produces billions of cells each day. This challenge is greater in developing individuals than in adults because the marrow must produce enough new cells to maintain a stable cell number per body mass as the baby grows. Preterm birth or illness in the newborn period is associated with additional hematologic stressors, which can result in anemia or neutropenia. This chapter addresses multiple mechanisms of anemia and neutropenia in the newborn and reviews the use of available recombinant growth factors to treat these conditions.
Erythropoietin
Physiologic and Pharmacologic Effects
Erythropoietin (Epo) is an endogenous glycoprotein that regulates erythrocyte production (1,2). Since the Food and Drug Administration (FDA) approval in 1989, many trials have been done (and reviewed) to test the safety and efficacy of recombinant human (r)Epo as an erythropoietic agent in neonates (3,4,5). rEpo is now widely used to treat or prevent anemia due to a variety of causes including renal failure and prematurity. Compared with adults, neonates require higher doses of rEpo per kilogram and more frequent dosing to achieve an equivalent hematopoietic response, due to their greater plasma clearance, high volume of distribution, and short fractional elimination time (6,7,8).
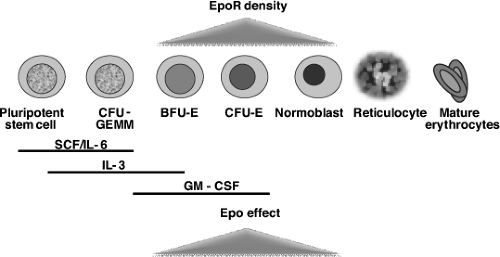
Humans have four main sites of embryonic and fetal erythropoiesis: yolk sac, ventral aspect of the aorta, liver, and bone marrow. Epo production mirrors this, beginning in the yolk sac, moving to the liver as the primary source during most of fetal life, and then switching to renal production around the time of birth. Factors regulating this switch are still not fully understood (9,10). Growth factors important for definitive erythropoiesis include Epo, stem cell factor (c-kit ligand), interleukin-3 (IL-3), IL-6, granulocyte–macrophage colony-stimulating factor (GM-CSF), and possibly insulin and insulin-like growth factor 1 (IGF-1), both of which act as nonessential survival factors for CD34+ cells (11,12). Epo maintains red cell production during fetal, neonatal, and adult life by inhibiting apoptosis of erythroid progenitors and by stimulating their proliferation and differentiation into normoblasts (13,14). Epo receptor (EpoR) density is highest in the burst-forming units erythroid and colony-forming units erythroid (Fig. 55.1). In addition to increasing and maintaining erythrocyte progenitors, Epo increases the synthesis of hemoglobin, membrane proteins, and transferrin receptors. Epo can be considered the primary growth factor in the process of erythropoiesis, as in its absence, definitive erythropoiesis does not occur: both Epo and EpoR null mutation mice die on the 13th day of intrauterine development due to absence of secondary erythropoiesis (15). Since Epo does not cross the placenta, Epo concentrations measured in the fetus reflect fetal synthesis (16).
During development, the EpoR is present on many nonhematopoietic cell types, including liver stromal cells (17), smooth muscle cells (18), myocardiocytes (19), endothelial cells (20), enterocytes (21), renal tubular cells, epithelial cells in the lung, retinal cells (22), placental tissues (23), Leydig cells (24), and cells specific to the central nervous system (25,26,27). The role of Epo in these tissues is under investigation.
To maintain the increase in red cell volume associated with fetal growth, it is estimated that approximately 50 ÷ 109 erythrocytes per day must be produced. When compared with adult Epo concentrations present at the time of acute anemia, measured fetal Epo concentrations seem low in the face of such production requirements. It has, therefore, been proposed that Epo is more efficient in the stimulation of the erythropoiesis during fetal development, that it acts as a paracrine factor during hepatic hematopoiesis, and/or that other growth factors synergize with Epo. Candidate factors include hepatic growth factor, thrombopoietin, and IGF-1 (28,29). Production of Epo is stimulated by hypoxia-inducible factor 1 and 2 and is regulated by requirements for tissue oxygenation. Elevated Epo concentrations (up to 8,000 mU per mL) have been reported in pathologic states, such as fetal hypoxia, anemia, and placental insufficiency, and in infants of diabetic mothers (30,31).
In healthy term infants, serum Epo concentrations decrease following birth to reach a nadir between 4 and 6 weeks of life. By 10 to 12 weeks of life, they reach adult concentrations (approximately 15 mU per mL). In preterm
infants, the fall in Epo is more profound and persists longer, contributing to anemia of prematurity.
infants, the fall in Epo is more profound and persists longer, contributing to anemia of prematurity.
Clinical Trials in Newborn Infants
Preterm infants remain among the most highly transfused patient populations despite attempts to limit phlebotomy losses, the implementation of transfusion guidelines, and the use of rEpo. Common contributors to anemia in the hospitalized preterm infant include phlebotomy loss (which may exceed the infant’s circulating blood volume), short red blood cell life span (70 vs. 120 days in adults), high growth requirements, iron deficiency, inflammatory states, and anemia of prematurity. When measured, circulating Epo concentrations in this population are low relative to the degree of anemia (32). Other forms of anemia in neonates include Rh-hemolytic disease of the newborn and a variety of anemias that are associated with chronic lung disease. The role of rEpo administration has been tested in each of these conditions, and also in neonates with congenital heart disease where an elevated hematocrit is desired.
Anemia of Prematurity
The majority of erythrocyte transfusions administered to very low-birth-weight (VLBW, birth weight <1,500 g) neonates are given during the first 3 weeks of life (33). The use of rEpo to avoid excessive transfusions in preterm infants has been studied in many randomized controlled trials. Several reviews have evaluated the safety and efficacy of rEpo treatment (3,5,34). rEpo treatment used together with iron is safe, well tolerated, and decreases both the number of transfusions and the volume of blood transfused when used at doses of greater than 500 U per kg per week. However, rEpo treatment may not prevent all transfusions or even significantly decrease donor exposures. This is because clinical practice is so variable: transfusion guidelines differ in stringency, phlebotomy practices vary by institution, and the timing and dose of both rEpo and iron vary widely.
One reasonable approach to managing anemia in the extremely preterm infant is to combine the use of iron supplementation, blood transfusion, and rEpo therapy with the goal of one donor exposure per infant maximum. Optisol®-preserved blood can be stored for up to 42 days. One adult unit of blood can be divided into aliquots and assigned to one infant. The infant can be transfused with these aliquots as needed during the first 6 weeks of life (one donor exposure). Iron status should be optimized. rEpo with iron can be used to prevent further transfusions if the infant remains significantly anemic with low reticulocyte counts. Judicious phlebotomy practices should be implemented.
Hyporegenerative Anemia of Neonates with Rh-Hemolytic Disease
Infants with Rh-hemolytic disease can develop a late anemia at 1 to 3 months of life secondary to diminished erythrocyte production. The incidence of late anemia seems to be much higher in infants who receive intrauterine transfusions (35,36,37). In these infants, the anemia is characterized by low plasma concentrations of Epo, while erythroid progenitors remain highly responsive to rEpo in vitro (37). Studies evaluating the use of rEpo as a treatment for neonates with late hyporegenerative anemia have shown mixed results (35,38,39).
Anemia of Bronchopulmonary Dysplasia
The anemia associated with bronchopulmonary dysplasia is normocytic, normochromic, and hyporegenerative with marrow normoblast iron stains that are distinct from those observed in the anemia of chronic disorders and the anemia of prematurity (40). In a study by Ohls et al., neonates with the anemia of bronchopulmonary dysplasia received 200 U per kg of rEpo per day subcutaneously for 10 consecutive days. Infants treated with rEpo at this dose and duration had increased reticulocytes and hematocrits and required fewer transfusions than placebo recipients (41).
Neonates with Congenital Heart Disease
Infants with certain varieties of congenital heart disease often experience prolonged hospitalization, multiple invasive procedures, and significant phlebotomy losses. These neonates frequently receive multiple blood transfusions. Neonates with congenital heart disease awaiting heart transplantation who received 200 U per kg per day of rEpo
had significant increase in hematocrit and a decrease in transfusions (42).
had significant increase in hematocrit and a decrease in transfusions (42).
Only a limited number of studies have evaluated the role of rEpo as an alternative to transfusions in neonates awaiting cardiac surgery. In Japan, a study evaluated the effect of three doses of rEpo (300 U per kg) on transfusion requirements of infants undergoing cardiac surgery (43). A beneficial effect of rEpo has been reported among neonates who underwent open heart surgery and in those whose parents are Jehovah’s Witnesses (44). Further studies evaluating the use of rEpo to reduce transfusion requirements in neonates with congenital heart disease are needed.
Structure, Dose, Routes, and Regimens
Recombinant human Epo is produced in Chinese hamster ovary cells by recombinant DNA technology, as a 165-amino acid glycoprotein with a molecular weight of 30.4 kDa. The human recombinant form of Epo is commercially available as epoetin alfa (rEpo) (Epogen, Amgen, Thousand Oaks, CA; Procrit, Ortho Biotech, Raritan, NJ).
A novel erythropoiesis-stimulating protein, Darbepoetin alpha (Aranesp), is a protein closely related to rEpo. It is also a 165-amino acid glycoprotein but contains five N-linked oligosaccharide chains, rather than the three contained in rEpo (45). These two additional glycosylation sites increase the molecular weight to 37.0 kDa and increase the terminal half-life threefold as determined in adult patients (46). Aranesp is available from Amgen and is formulated for intravenous and subcutaneous administration.
A wide range of dosing schedules have been used for hyporegenerative anemia in the preterm infant (50 to 700 U per kg per dose) (5,47,48). Garcia et al. showed that for every 500 U per kg per week increase in rEpo dosing, the average number of transfusions per patient decreased by three-fourth of a transfusion (5). We recommend subcutaneous administration of rEpo 400 U per kg three times per week, or daily intravenous rEpo 200 U per kg per day, for a minimum of 2 weeks (49). Alternatively, rEpo can be administered in a continuous intravenous infusion such as parenteral nutrition, using a dose of 200 U per kg per day (50). To promote effective erythropoiesis, iron must be given concomitant with rEpo. Patients on full enteral feedings who are receiving rEpo should receive 6 to 8 mg per kg per day of elemental iron. Alternatively, parenteral iron may be given at 1 mg per kg per day (49). Zinc protoporphyrin to heme ratios (ZnPP/H) may be followed weekly to assess and optimize iron status (51,52). Serum ferritin levels may also be helpful in assessing iron stores.
Aranesp has the advantage of a single once-weekly injection rather than the thrice-weekly dosing of rEpo (45). This more highly glycosylated formulation has now been well studied in adults, but there are few studies in preterm or term infants regarding the pharmacokinetics, efficacy, risks, or benefits of Aranesp in neonates (53).
Pharmacokinetic Properties in Newborn Infants
rEpo can be administered by intravenous infusion or subcutaneous injection. Pharmacokinetic studies in preterm infants performed by Ohls et al. revealed that there were no differences in plasma Epo concentrations at day 3 and day 10 or in the rate of clearance between intravenously and subcutaneously administered rEpo (50). In the same study, the elimination half-life of rEpo was 17.6 ± 4.4 hours on day 3 and 11.2 ± 1.5 hours on day 10. When compared with pharmacokinetic studies performed in adults, rEpo administered to preterm infants has a three- to fourfold increase in both volume of distribution and clearance (7,50,54). The precise mechanism for Epo elimination has not been established in neonates, but it has been speculated that rEpo is eliminated by irreversible binding to its receptors on erythroid progenitor cells with subsequent internalization. Renal excretion of Epo is reported to be less than 2% of the total (nonsignificant) in adult subjects.
Adverse Effects, Toxicity, and Precautions
Known adverse effects of chronic rEpo treatment in adults include hypertension (55), thrombus formation (56), polycythemia, and red cell aplasia secondary to anti-Epo antibodies (57). Hypertensive leukoencephalopathy has also been reported in a few adult patients requiring long-term rEpo for anemia due to dialysis-dependent renal failure (58). An FDA warning was released in 2008 for patients with chronic kidney failure who receive rEpo at higher than recommended doses, as an increased risk of blood clots, strokes, heart attacks, and death have been identified. In preterm neonates, rEpo use for the treatment of anemia has been very safe with none of the adverse effects noted in adult populations (59).
One potential adverse response is unique to preterm infants. Retinopathy of prematurity (ROP) is a neonatal disease characterized by the pathophysiologic growth of immature blood vessels across the entire retina, which can trigger retinal detachment and loss of vision if unchecked (60). The disease primarily affects low-birth-weight (<1,250 g) and preterm infants born less than 31 weeks’ gestation (61). Retinal vascularization occurs from 16 weeks’ gestation to birth (62) and in infants born prematurely, this process can be disrupted. ROP occurs in two phases, the first involving a loss of retinal vasculature following birth and the second involving uncontrolled proliferation of retinal vessels. EpoRs are present on endothelial cells, and rEpo stimulation increases their angiogenic expression (20). Early high-dose rEpo may have a protective effect on the retina by ameliorating the first stage of ROP (63). Alternatively, the angiogenic properties of Epo may prevail, resulting in an increase in ROP, particularly if the timing of dosing coincides with second phase of ROP (63). An increased risk of ROP was identified in one meta-analysis of early use of rEpo and iron for erythropoiesis (relative risk 1.71, CI 1.15 to 2.54) (3). It is not clear whether this is an effect of rEpo or of early iron administration, since rEpo was used in conjunction with iron. Many other studies have shown no such association. No prospective randomized controlled studies have been done to study this issue.
Nonhematopoietic Effects of Erythropoietin
Following the observations of EpoR expression in brain and other organs (22,27) and the capacity for Epo production
by astrocytes (64), a broader concept of Epo as a neuroprotective molecule has emerged (65). Neuroprotection by high-dose rEpo has been demonstrated in neonatal and adult animal models of injury including hypoxia-ischemia, stroke, and hemorrhage (66,67,68). rEpo improves both short- and long-term outcome following unilateral neonatal brain injury in neonatal rats, decreasing structural and behavioral deficits (67,69,70,71,72,73). Brief courses (5 days) of high-dose rEpo have also been shown to be safe in neonatal rats with no long-term negative consequences (74). Thus, rEpo may provide an important adjunct to neuroprotective therapy in neonates. The mechanisms by which rEpo provides neuroprotection are complex and include direct neuronal effects and indirect systemic effects, as well as both early and late effects. Early effects of rEpo include antiapoptotic (75,76,77), anti-inflammatory (68,78), and antioxidant effects (68,76,79,80,81,82) and increased resistance to excitotoxicity (83,84). Late rEpo effects that improve brain recovery include increased neurogenesis, angiogenesis, and migration of regenerating neurons (85,86). rEpo is directly involved in prevention of oxidative stress with generation of antioxidant enzymes, inhibition of nitric oxide production, and decrease of lipid peroxidation (87). These properties of rEpo may be relevant in therapeutic prevention of injury in developing brain of premature infants, where antioxidant systems are immature. Inflammation is also an important component in the pathogenesis and progression of both preterm and term brain injury. Since rEpo has a long track record of use in preterm infants and systemic high-dose rEpo can cross the injured and intact blood–brain barrier (88,89), it has promise as a neuroprotective agent in this population.
by astrocytes (64), a broader concept of Epo as a neuroprotective molecule has emerged (65). Neuroprotection by high-dose rEpo has been demonstrated in neonatal and adult animal models of injury including hypoxia-ischemia, stroke, and hemorrhage (66,67,68). rEpo improves both short- and long-term outcome following unilateral neonatal brain injury in neonatal rats, decreasing structural and behavioral deficits (67,69,70,71,72,73). Brief courses (5 days) of high-dose rEpo have also been shown to be safe in neonatal rats with no long-term negative consequences (74). Thus, rEpo may provide an important adjunct to neuroprotective therapy in neonates. The mechanisms by which rEpo provides neuroprotection are complex and include direct neuronal effects and indirect systemic effects, as well as both early and late effects. Early effects of rEpo include antiapoptotic (75,76,77), anti-inflammatory (68,78), and antioxidant effects (68,76,79,80,81,82) and increased resistance to excitotoxicity (83,84). Late rEpo effects that improve brain recovery include increased neurogenesis, angiogenesis, and migration of regenerating neurons (85,86). rEpo is directly involved in prevention of oxidative stress with generation of antioxidant enzymes, inhibition of nitric oxide production, and decrease of lipid peroxidation (87). These properties of rEpo may be relevant in therapeutic prevention of injury in developing brain of premature infants, where antioxidant systems are immature. Inflammation is also an important component in the pathogenesis and progression of both preterm and term brain injury. Since rEpo has a long track record of use in preterm infants and systemic high-dose rEpo can cross the injured and intact blood–brain barrier (88,89), it has promise as a neuroprotective agent in this population.
Epo may also function as a paracrine–autocrine tissue protective hormone in other organs such as heart and kidney (90). The mechanism of rEpo action in these organ systems is thought to be similar to neuroprotective mechanisms (91,92). Clinical trials are in progress to test these hypotheses.
Clinical Studies of Neonatal Neuroprotection with Erythropoietin
Two pilot studies evaluating the safety of high-dose rEpo in preterm populations have been published (93,94). The doses included in these studies ranged from 500 to 3,000 U per kg per dose given daily for three doses. Larger randomized controlled trials to evaluate the efficacy of high-dose rEpo in preterm infants are in the planning stage, or ongoing.
Erythropoietic doses of rEpo may also be protective; however, we currently have insufficient evidence to determine this. One study reported on the long-term neurodevelopmental effects of erythropoietic doses of rEpo: VLBW infants were randomized to rEpo or control treatment from day 4 of life until 35 weeks of corrected gestational age. There were no differences in neurodevelopmental outcomes at 18 to 22 months (95). In contrast, in preterm infants weighing less than 1,000 g treated with rEpo (400 U per kg three times per week) for a similar duration, those with serum Epo concentrations greater than 500 mU per mL, had higher mental development index scores than infants with Epo concentrations of less than 500 mU per mL when tested at 18 to 22 months corrected age (n = 6 rEpo, n = 6 placebo/control) (96). This suggests that higher circulating Epo concentrations may be of benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree