: Elements of bone. (1) Multinucleated osteoclasts; (2) Osteoblasts; (3) Osteoid; (4) Lining cells; (5) Mineralized bone
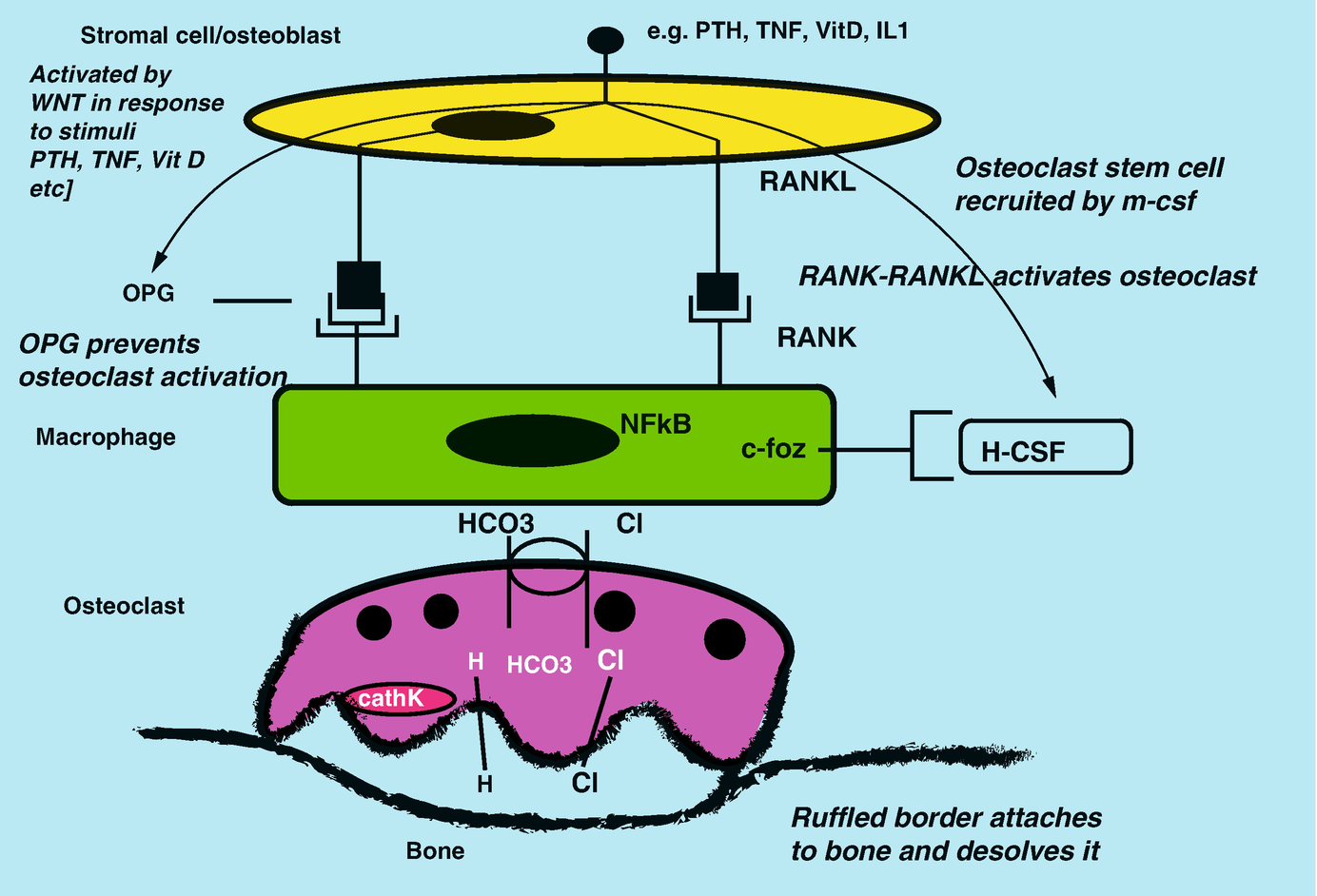
Interactions between osteoblast and osteoclast precursors and activation
Bone-preserving OPG is upregulated by estrogen, vitamin D, calcium, and TNF-a. It is suppressed by PTH, glucocorticoids, and immunosuppressants leading to bone resorption. Changes in these stimuli and hormones will result in perturbations of bone activity leading to abnormal bone formation and fragile bone.
The Osteoclast
Osteoclasts are from hematopoietic origin, the same stem cells that give rise to the monocyte and macrophage lineage (Fig. 18.1). The osteoclast precursors are activated by circulating macrophage differentiation factors (mCSF) and OPG from osteoblasts via the activation of the RANK pathway. The multinucleated osteoclasts are tasked to degrade bone and associated proteins. They attach to the calcified bone via their ruffled border and produce bone-degrading enzymes and acid such as cathepsin K and hydrochloric acid (Fig. 18.2).
These cellular systems interact with each other in a coupled process called bone turnover, so as to maintain balance between bone formation and bone resorption. Bone turnover maintains structural integrity with cortical bone and metabolic activity with trabecular bone. The normal adult skeleton turns over or remodels 10% yearly [10].
Bone serves multiple purposes. It provides structure, support, and protection as well as a major metabolic platform. Structure and support are attributed to the cortical bone, the calcified layer containing mature osteocytes encased in a calcified matrix. Once considered inactive, it is now known that the osteocytes respond to a number of biochemical and mechanical stimuli and communicate with each other through a network of fluid-filled canaliculi [11]. These osteocytes may be involved in mineral homeostasis [8] as well as bone fracture repair. Metabolic support is afforded by the trabecular bone. This scaffolding, also contributing to structural support, is composed of bone-forming cells and their matrix products. The trabecular bone responds promptly to changes in metabolic status, helping to maintain mineral homeostasis.
Chronic Kidney Disease and Bone Health in the Woman with Renal Disease
Bone health is defined by bone mass and bone quality. Peak bone mass is achieved by age 30. Bone quality is affected by clinical status. Pregnancy, menopause, chronic kidney disease, and organ transplant status all affect bone turnover and architecture.
Effect of Estrogen on Bone
During pregnancy, higher levels of estrogen may initially increase maternal bone mass [12]. This is followed by increased calcitriol production, elevated PTHrp and prolactin (for lactation), and increased calcium in breast milk, needed for the baby’s growing skeleton. This leads to calcium leaching from maternal bones and a discrete loss of bone mineral density (BMD). Estrogen levels subsequently decrease; osteoclast activity increases, leading to increased bone turnover. This status is reversed upon weaning [13].
As menopause sets in, bone turnover increases as estrogen levels decrease with loss of OPG inhibitory effect on osteoclast activation. Bone mass loss is most evident at trabecular sites. It is reported that there is a bone loss of 9.1% in the femoral neck and 10.6% at the vertebral spine [14]. There is attenuated but continued bone loss after the first 10 years of menopause. Thus, women are very vulnerable to debilitating bone fractures.
Chronic kidney disease is associated with unique metabolic bone disease (MBD). As renal function declines, a number of hallmark metabolic processes are altered. Calcitriol generation decreases as renal mass declines leading to parathyroid hormone disturbances. The fracture incidence in dialysis and CKD Stage 1–4 patients is much higher than in the general population [3, 4]. In addition to effects from MBD, the higher fracture rate can be attributed to the fact that these patients are living longer [15] and have the added contribution from aging, low hormone levels, elevated fall rates, and malnutrition.
High Turnover Bone Disease
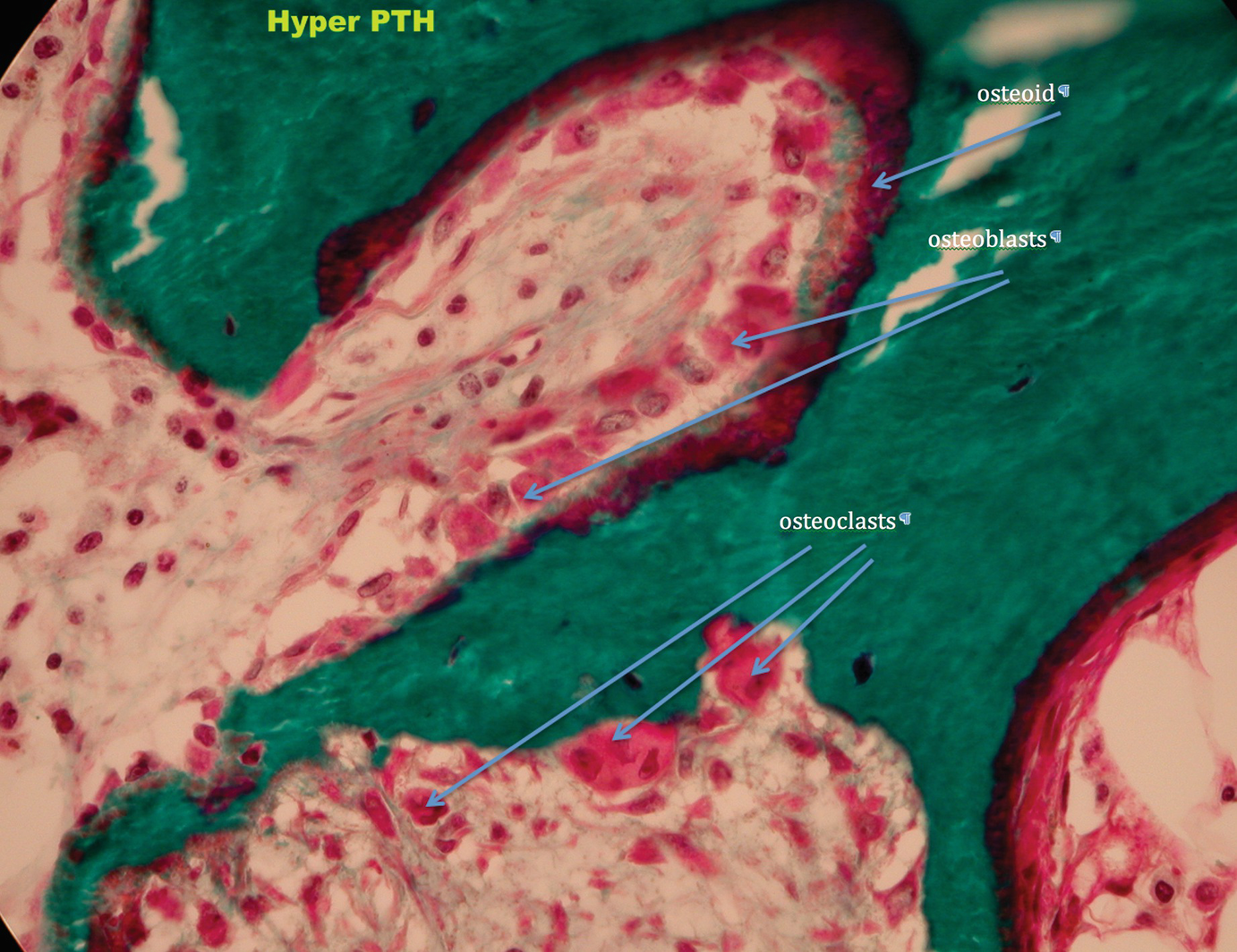
Hyperparathyroid bone disease
Low Turnover Bone Disease
Alternatively, bone turnover may decline to almost zero, depending on the underlying disease processes or to various treatments the patient has or is receiving.
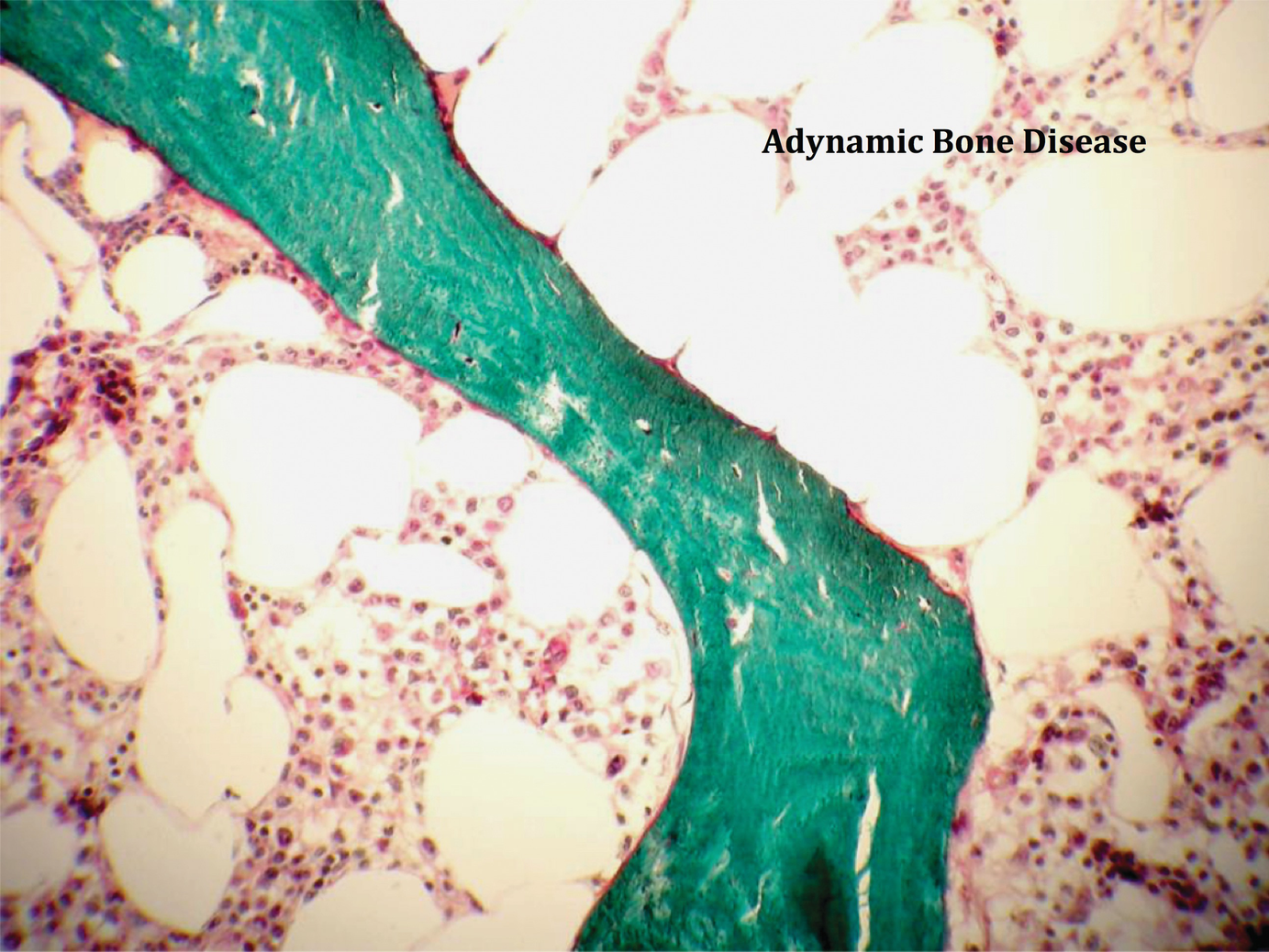
Adynamic bone disease with paucity of cellular elements and activity
Renal Transplantation
All patients undergoing renal transplantation have underlying metabolic bone disease. The renal transplant regimen adds another layer of complexity to transplant bone disease. Glucocorticoids, although minimized in modern regimens, suppress osteoblast activity by inhibition of growth factors and bone morphogenic protein. They suppress sex steroids, decrease gastrointestinal calcium absorption, and increase urinary calcium excretion. Parathyroid hormone (PTH) is stimulated with continued use of glucocorticoids. Calcineurin inhibitors (CNI) may directly stimulate secretion of PTH, further contributing to increased bone turnover and trabecular weakness [17]. Cyclosporine may preferentially stimulate osteoclast activity [18]. Tacrolimus may induce osteoblast differentiation leading to increased osteoclast production and high turnover [19, 20]. CNIs can adversely affect allograft function and contribute to chronic renal dysfunction, with accompanying levels of hyperparathyroidism further negatively affecting bone. Of note, CNIs likely contribute to metabolic bone disease associated with other solid organ transplants as they can lead to renal disease in the native kidney of the transplanted patient [21].
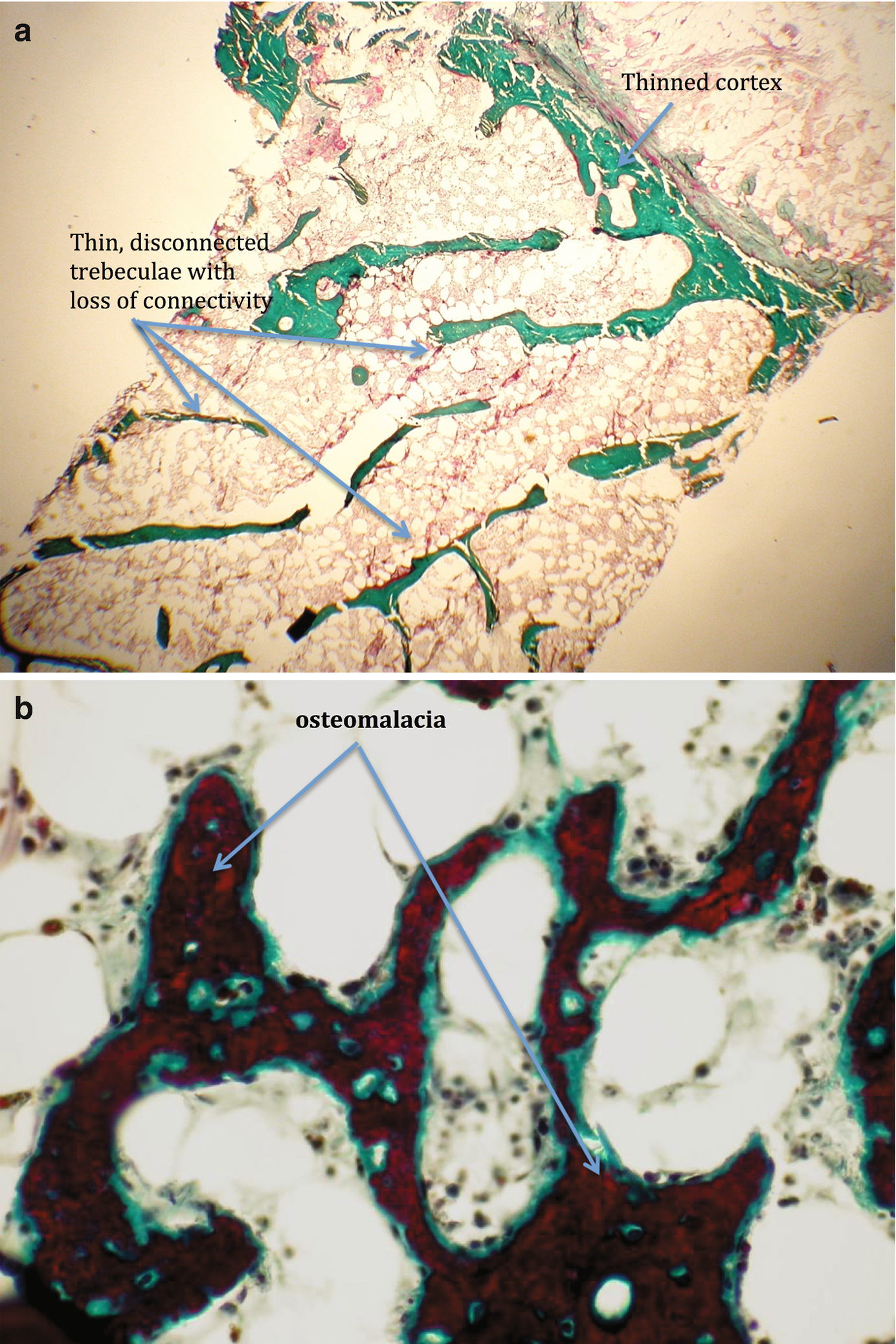
One cannot predict the underlying bone disease in women with CKD undergoing renal transplantation. They may have high bone turnover disease (hyperparathyroid disease) or low bone turnover disease (adynamic bone disease or osteomalacia) [22, 23]. Almost all of these women are menopausal given their low pretransplant estrogen status [22], and many will have been treated with bone modulators during renal replacement therapy including vitamin D, cinacalcet, or phosphate binders. They are then exposed to immunosuppression therapy with varying doses of glucocorticoids and CNIs. It is not surprising that they tend to lose bone mass in the first year following transplant. While patients are no longer routinely exposed to high maintenance steroid therapy and so are not losing the large percentage of BMD described in the early days of transplantation [24], they are still losing bone mass. Even in the current age of renal transplantation with steroid sparing immunosuppressive agents, bone mass does decrease, although the risk of bone fractures is not as high as it historically was. However, many centers and nephrologists try to attenuate the risk of fracture by empiric treatment with various bone modulators, mainly bisphosphonates. BMD often does not improve with this therapy.
Recent studies have shown that underlying bone disease in these patients is not uniform. Bone histomorphometric studies show a predominance of adynamic bone disease [22, 25]. In this situation, bone suppressive therapy is not indicated and may be detrimental as bone turnover is suppressed further.
Sex hormones are very low at the beginning of renal transplant, but they tend to improve in younger women after several months to 1 year posttransplantation perhaps impacting positively on BMD.
Evaluating Bone Health
Bone Mineral Density

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


