Growth and Metabolic Adaptation of the Fetus and Newborn
Ricardo Uauy
Patricia Mena
Joseph B. Warshaw
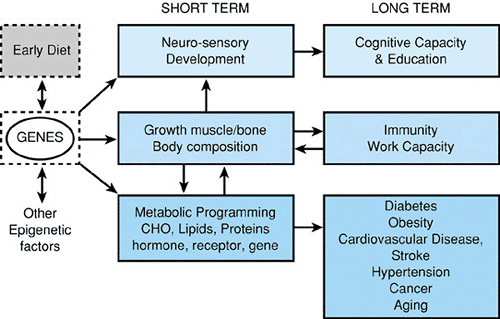
Recent developments in understanding the fetal origins of adult health demonstrate the importance and complexity of the interaction of genetic and epigenetic factors with nutrients, hormones, substrates and toxicants in determining health and disease throughout life. The task of pediatricians is not limited to childhood. It is increasingly to promote health and prevent disease from the moment of conception onward (Fig. 20.1).
GENETIC AND ENVIRONMENTAL INFLUENCES ON FETAL GROWTH
Genetic Influences
Genetic composition has a profound influence on fetal growth. This is perhaps most clearly demonstrated by the effects of an abnormal number of chromosomes (aneuploidy). Turner syndrome (45 X0), trisomy 21, trisomies 13 and 18, triploidy, and polyploidy are all associated with poor fetal growth, and experimental studies have shown slow cell division in trisomic or triploid cell lines. Aberrant fetal growth also accompanies nonaneuploid disorders. Hereditary gigantism (Sotos syndrome) and the genetic forms of dwarfism represent extremes. Uniparental disomy (abnormal parent-of-origin genetic expression) of chromosome 7 is associated with poor growth in Silver-Russell syndrome, whereas a similar abnormality of chromosome 11 leads to fetal overgrowth in Beckwith-Wiedemann syndrome.
Genetic influences play a role in the birth weight variability observed among ethnic groups, ranging from a mean birth weight of 2,400 g in pygmies to a mean of 3,500 g or greater in affluent subpopulations of industrialized countries (Box 20.1). The effect of genotype also is evident in the greater birth weight among males, averaging 150 g more than females at term.
Uterine and Placental Influences
Environmental influences on fetal growth include uterine blood flow, placental function, and local uterine, placental, and umbilical circulation. Taken together, these determine the substrate and gas flux available to the fetus. In general, the placenta and baby grow proportionately; large babies have large placentas and small babies, small placentas, with the placenta weighing about 20% of the baby’s weight. Conditions that compromise placental size and function, such as abnormal uterine anatomy, ectopic placental insertion, placental abruptio or
infarction, placental hemangioma or arteriovenous fistulae, congenital infections, and abnormal cord insertion may adversely affect fetal growth. Decreased placental blood flow measured with Doppler ultrasound undoubtedly plays a role in the intrauterine growth retardation associated with maternal toxemia, severe diabetes and/or long standing hypertension, and tobacco smoking.
infarction, placental hemangioma or arteriovenous fistulae, congenital infections, and abnormal cord insertion may adversely affect fetal growth. Decreased placental blood flow measured with Doppler ultrasound undoubtedly plays a role in the intrauterine growth retardation associated with maternal toxemia, severe diabetes and/or long standing hypertension, and tobacco smoking.
BOX 20.1 Genetic, Hormonal, and Environmental Influences on Fetal Growth
Genetic and fetal factors
Species, race, gender
Congenital anomalies
Chromosomal disorders
Fetal hormones (insulin, corticosteroids, thyroid hormone, androgens)
Growth factors (IGF I and IGF II, EGF and TGF-1)
Maternal uterine environment
Uterine and placental anatomy
Utero-placental function
Human placental lactogen
Substrate fluxes and transfer
Uterine blood flow
Maternal systemic disease
Macroenvironment
Infectious agents (STORCHa)
Diet and nutrition
Social and emotional stress
Drugs and smoking
Teratogens and toxins
Altitude and temperature
Ionizing radiation
Footnote
aSTORCH, syphilis, toxoplasmosis, rubella, cytomegalovirus, herpesvirus.
Placental influences in addition to blood flow and placental surface area include placental hormones and growth factors. Their effects may be mediated indirectly through modification of blood and substrate flow or directly by regulation of cell replication and differentiation.
Maternal Nutrition
The maternal nutritional environment is critical for fetal growth. Although the mother tends to buffer the effect of adverse environmental conditions on the fetus, maternal weight gain during pregnancy is positively correlated with infant birth weight. The classic studies of the Dutch famine during World War II showed a mean birth weight reduction of 300 g among infants whose mothers suffered severe caloric deprivation during the last trimester of gestation. In previously well nourished mothers, caloric deprivation must be extreme before fetal growth is compromised. However, in women from developing countries, where malnutrition is entrenched over generations, a moderate energy deficit will have an adverse effect. In this regard, studies have shown that maternal height, in part the result of early nutritional influences, has a positive association with birth weight. After 2 years of age, the growth of the infant correlates better with mean parental height rather than maternal height alone; genetic factors contributed by both parents are important in determining final size, whereas early maternal nutrition, reflected in maternal height, is the major determinant of fetal growth. The effects of maternal size may be multigenerational. Mothers who were small for gestational age (SGA) at birth are at greater risk of having an SGA or preterm baby. These effects may be mediated through the size of the uterus and its capacity to hypertrophy and increased blood flow in response to pregnancy.
First-born infants on average weigh less than subsequent infants. Mothers less than 15 and over 35 years of age have a higher incidence of low-birth-weight babies, only partially explained by parity and socioeconomic risk factors. Maternal nutrition, especially in adolescents, and uterine and placental factors, are thought to play a role.
The practical consequence of these findings is that the evaluation of fetal growth should consider infant sex, maternal height, and birth order in addition to gestational age.
Micronutrients
Micronutrient minerals and vitamins are increasingly recognized as agents that affect embryogenesis and the incidence of congenital malformations.
Folate intake before and during early embryogenesis alters the incidence of neural tube defects (NTDs). The involvement of a genetic component for NTDs is evident in the high rate of recurrence in families and individual mothers. NTDs are also more frequent in certain ethnic groups. If NTDs were solely genetic, prevalence should not vary over time, yet NTDs are more frequent in periods of nutritional deprivation, such as during the 1945 Dutch famine. These observations led to the discovery of the striking benefit of maternal administration of doses of 400 μg of folic acid. Folic acid from food sources must be reduced to tetrahydrofolic acid before it is metabolically active. Genetically determined defects in the responsible enzyme, methylenetetrahydrofolate reductase, have been found in 35% to 50% of mothers bearing children with NTDs, thus accounting for the protective effect of folate supplementation. Heterogeneity in receptor-mediated folate transport may explain susceptibility to NTDs unrelated to reductase activity.
Retinoic acid, derived from retinol (vitamin A), is a regulator of gene expression and a teratogen early in embryonic development. Of interest, retinoic acid decreases the risk of spina bifida in animals. The folate receptor gene is a target for retinoic acid transcriptional regulation, providing a possible explanation for folate-retinol interaction. The regulation of folate receptors may explain the occurrence of NTDs in association with low vitamin A intake.
Maternal zinc deficiency has been implicated in abnormal fetal growth and enhanced susceptibility to such teratogens as alcohol, valproic acid, and arsenic.
Interaction of Genes and Diet
The beneficial effect of folic acid is only one example of the relationship between genetic composition and diet. The interaction of genes and the early diet not only determines brain development, growth, and body composition but also the later prevalence of nutrition-related chronic disease and some types of cancers. Genes are differentially expressed depending on the exposure to the epigenetic nutrients and toxicants. Thus, a similar genotype may define multiple phenotypes. The regulation of gene expression can occur at multiple levels. Nutrients can bind to specific or nonspecific ligands that interact with response elements in DNA. Nutrients may change the phosphorylation status of a protein and thus its activity. At the post-transcriptional level, nutrients may modify native RNA processing, messenger RNA (mRNA) transport and stability, and breakdown rates. Nutrients may modify the rate of mRNA translation. Finally, nutrients can modify the turnover rates of enzymes and other proteins, thus affecting their activity level.
Maternal Medical Disorders
Preeclampsia, chronic hypertension, collagen vascular disease, and renal disease all affect fetal growth by compromising maternal nutritional status and interfering with uterine and placental perfusion. Severe maternal anemia and diminished cardiac output secondary to heart disease and/or cyanotic congenital heart disease affect fetal growth by decreasing oxygen availability to the maternal uterine compartment. Early abnormalities in embryonic fuel metabolism in prediabetic or diabetic mothers may play a role in determining abnormal fetal growth and may also be teratogenic; later, maternal hyperglycemia induces fetal hyperinsulinism and macrosomia with enhanced growth of peripheral adipose tissue, muscle hypertrophy, and increased liver glycogen stores. The goal of health care and nutrition before and during pregnancy must be to prevent both intrauterine growth restriction (IUGR) and macrosomia.
Intrauterine infections, including rubella and cytomegalovirus infections, can impair fetal growth. Toxoplasmosis, syphilis, and herpes infections, although less frequent during the first trimester, may affect fetal growth by arresting cell replication during critical stages of development, causing typical patterns of malformations and severely compromised growth.
Other Influences on Fetal Growth
Altitude is associated with diminished fetal growth due to lower ambient oxygen tension. Radiation exposure has been
associated with microcephaly and abnormal fetal growth. Organic solvents and heavy metals, especially mercury and cadmium, have been associated with malformation and compromised fetal growth. Smoking, especially in the last trimester of pregnancy, reduces birth weight and length; the effect is proportional to the number of cigarettes smoked. Pre- and postnatal growth failure and microcephaly characterize the fetal alcohol syndrome. Growth restriction occurs in infants born to mothers addicted to heroin, cocaine, or methadone. Other drugs with adverse affects include anticonvulsants (phenytoin [Dilantin], phenobarbital, and carbamazepine [Tegretol]), antifolates (methotrexate), warfarin (Coumadin), and prednisone.
associated with microcephaly and abnormal fetal growth. Organic solvents and heavy metals, especially mercury and cadmium, have been associated with malformation and compromised fetal growth. Smoking, especially in the last trimester of pregnancy, reduces birth weight and length; the effect is proportional to the number of cigarettes smoked. Pre- and postnatal growth failure and microcephaly characterize the fetal alcohol syndrome. Growth restriction occurs in infants born to mothers addicted to heroin, cocaine, or methadone. Other drugs with adverse affects include anticonvulsants (phenytoin [Dilantin], phenobarbital, and carbamazepine [Tegretol]), antifolates (methotrexate), warfarin (Coumadin), and prednisone.
INTERACTION OF NUTRIENTS, HORMONES, AND GROWTH FACTORS DURING PERINATAL GROWTH
Fetal growth depends on adequate nutritional substrates and on the action of insulin and growth factors such as epidermal growth factor and IGF-I and -II. Insulin regulates fetal lipogenic activity and has a permissive role in protein synthesis and hepatic glycogen deposition. Fetuses with insulin deficiency secondary to pancreatic agenesis or with a defective insulin receptor have marked IUGR with decreased adipose tissue and little weight gain during the last trimester of pregnancy (leprechaun syndrome). Conversely, fetal hyperinsulinism results in increased adiposity in human infants of diabetic mothers.
Protein feeding, as well administration of several essential and nonessential amino acids, stimulates insulin secretion in the fetus and neonate. Increasing arginine levels during parenteral infusion also has been shown to increase serum insulin levels. The correlation of urinary excretion of the insulin precursor C-peptide with weight gain suggests that insulin may be a growth-promoting factor for infants on high-protein diets. Preliminary evidence from controlled clinical studies in extremely small preterm infants has shown increased tolerance to glucose and higher weight gain in infants infused with insulin during their initial postnatal days.
Peptide growth factors that influence fetal growth and maturation include the insulin-like growth factors (IGF-I and -II). In the fetus, these act independently of growth hormone. IGF-I influences terminal differentiation of a number of tissues, including brain astrocytes, neural outgrowth, and myogenesis; and, although the influences of IGF-I appear to be local, serum concentrations of IGF-I correlate with birth weight. Both IGF-I and -II are complexed to binding proteins that modulate their biological activity. After birth, higher levels of IGF-I are observed in IUGR infants during catch-up growth, especially in association to gain in length. Epidermal growth factor (EGF) and transforming growth factor-1 (TGF-1) influence the growth and differentiation of epithelial cells in lung and gut. Receptors for EGF are present throughout development and are increased in number in the placenta and lung in fetuses with growth restriction induced by uterine artery ligation, thus suggesting a role for EGF in fetal growth retardation. Leptin is a circulating polypeptide hormone expressed by adipocytes and placenta. A positive relationship exists between fetal leptin concentrations and both gestational age and fetal weight.
Thyroxin and glucocorticosteroids have important influences on specific organ development and functional and metabolic adaptation, but relatively little influence on fetal somatic growth.
PLACENTAL TRANSPORT AND METABOLISM
The placenta transfers metabolic substrates and other nutrients from the mother to the rapidly growing fetus. In addition, the placenta allows for the excretion of fetal waste products and performs important metabolic and hormonal functions.
Anatomy
In the human placenta, the fetal villi are directly bathed by maternal blood; therefore, the fetal capillary circulation is separated from maternal blood by fetal connective tissue and the placental epithelium, composed of the cytotrophoblast and the syncytiotrophoblast. A clear understanding of placental ultrastructure is necessary in order to discuss the functional correlates. As shown in Figure 20.1, the uppermost layer of fetal tissues, the syncytiotrophoblast, is in direct contact with maternal blood. Microvilli increase the surface area necessary for transport. Syncytial vacuoles are responsible for the transport of macromolecules and may be specifically targeted by cell surface receptors. The extensive endoplasmic reticulum and the high density of mitochondria provide the anatomic basis for both synthetic activities and transport through the cytoplasm of the trophoblast. The multinucleated syncytiotrophoblast is derived from the actively replicating cytotrophoblast. The trophoblast represents the only uninterrupted cell layer interposed between the fetal capillary and maternal circulations.
The placenta grows at a very rapid rate during the initial stages of pregnancy. Placental growth is characterized by both increased numbers of villi and microvilli and proliferation of fetal capillary vessels. In this way, the surface area available for maternal–fetal exchange is greatly enhanced. Nutrients in maternal blood must cross the trophoblast cell layer and the basement membrane to reach the loose connective tissue surrounding the fetal capillaries (Fig. 20.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree