Essentials of Diagnosis
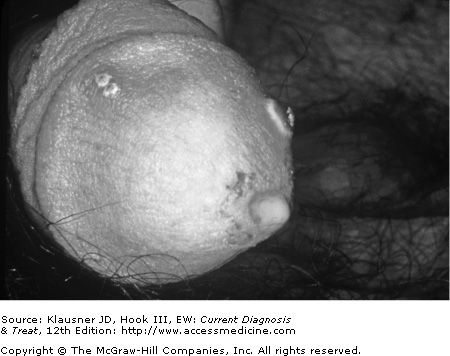
- • In men, findings include urethral discharge, urethral burning with urination, and swelling of the glans penis.
- • In women, findings include lower abdominal pain; cervicovaginal discharge, “spotting,” and bleeding; and pain with intercourse.
- • Disseminated infection produces a rash consisting of papules or pustules with an erythematous border; monoarticular arthritis or tenosynovitis may also be present.
- • Definitive diagnosis can be made by Gram stain, culture, molecular probe assay, or nucleic acid amplification testing.
General Considerations
Neisseria gonorrhoeae may cause several adverse health outcomes, including local genitourinary infections, upper reproductive tract disease, and disseminated gonococcal infections. An important impact of N gonorrhoeae infection is that it can facilitate acquisition of the human immunodeficiency virus (HIV) and shedding of HIV in semen.
The global incidence of gonorrhea infections remains high, with an estimated 62 million new cases each year. The highest rates occur in sub-Saharan Africa, south and Southeast Asia, the Caribbean, and Latin America. Although the overall prevalence of gonorrhea has declined in the United States since 1975, it remains the second most commonly reported communicable disease. Rates remain high in certain groups, including African-Americans and Hispanics, men who have sex with men (MSM), adolescents, and populations in the southeastern United States. Race in itself is not a risk factor for gonorrhea but may be a surrogate marker for socioeconomic and behavioral factors that increase the risk for infection.
Screening is an important tool for identifying and treating asymptomatic infection, the most common manifestation of gonorrhea, which serves as a source of ongoing community transmission. In addition to screening efforts directed at women to reduce the complications of untreated disease in this population, routine screening in specific risk groups, such as adolescents, MSM, or HIV-infected individuals is recommended.
Pathogenesis
The transmission of N gonorrhoeae following a single episode of vaginal intercourse has been estimated to be approximately 70–80% from male to female partners, and 20–30% from female to male partners. Transmission through either receptive or insertive rectal intercourse may be less efficient than vaginal intercourse but data are limited. Penile-oral and oral-penile transmission is probably the least efficient means of transmission but does occur; again, data on the true frequency of transmission are limited.
Following introduction into a mucosal site, gonococci attach to the surface of columnar epithelial cells and colonize mucosal cells through parasite-directed endocytosis. The exuberant host immune response to gonococcal infection, while leading to control of infection, can account for many of the signs and symptoms of disease.
Prevention
Behavioral interventions remain crucial strategies for reducing the spread of gonorrhea. These include delaying sexual debut and reducing the number of new partners, in addition to consistent condom use.
Consistent male condom use significantly reduces transmission of gonorrhea. A female polyurethane condom is at least as effective as male condoms, but major drawbacks to either type of condom include noncompliance and unacceptability.
All patients with gonorrhea should be retested at 3 months. Sexual contacts must also be identified and given treatment. In most instances, partners are asked to notify their sexual contacts of the need for screening and treatment. Expedited partner treatment programs that allow patients to take medication to their sex partners are increasingly common in many areas.
Research is ongoing in the development of vaginal and rectal microbicides or antimicrobial gels that can be used during sexual intercourse to prevent the transmission of infection.
Clinical Findings
The clinical manifestations of gonorrhea range from (most commonly) asymptomatic or mildly symptomatic disease to (more rarely) disseminated infection. The initial site of infection, infecting strain of N gonorrhoeae, host factors, and other coinfections all influence the spectrum of clinical disease experienced by the patient.
Although almost half of all gonococcal infections in men are asymptomatic or minimally symptomatic, gonococcal urethritis is the most common manifestation of symptomatic infection. The incubation period may range from 24 hours to 14 days, but most men develop symptoms (dysuria and discharge) within 4 days of infection (see Figure 16–1). Dysuria usually precedes the development of purulent discharge by approximately 24 hours. Untreated cases of gonococcal urethritis usually resolve over the course of several weeks, with the majority of cases becoming asymptomatic within 6 months.
Widespread availability of effective antimicrobial therapy since the 1940s has made rare most local genitourinary complications in men. Local complications include epididymitis, acute or chronic prostatitis, orchitis, seminal vesiculitis, posterior urethritis, and infection of the Tyson and Cowper glands.
Among MSM, the rectum is the only site of gonococcal infection in 40% of reported cases. Rectal infection may occur through direct inoculation by receptive anal intercourse and is usually asymptomatic. When symptoms occur, they are usually mild, producing only pruritus and painless rectal discharge. Rarely, more severe symptoms, including rectal pain and bloody mucopurulent discharge, may occur. External inspection may reveal few or no signs of infection, and anoscopy with collection of specimens for diagnosis is recommended (see Laboratory Findings, later). Without treatment the patient can carry the infection for months without symptoms.
Pharyngeal infection may occur in up to 10% of MSM who engage in oral-genital contact with an infected partner.
The incubation period for gonorrhea in women is usually less than 10 days. Approximately 50% of women have asymptomatic endocervical infection. Clinical presentation includes nonspecific vaginal discharge, abnormal menstrual bleeding, and anorectal discomfort. However, many other sexually transmitted diseases (STDs) in women present with the same symptoms.
Endocervical infection may be accompanied by urethral infection, with dysuria as a symptom. Gonorrhea can be mistaken for acute bacterial cystitis and should be suspected in young, sexually active women with pyuria in the absence of bacteruria. Some experts recommend that all women who present with dysuria should have pelvic and urethral examination for inflammatory exudates, with visualization of the cervix for signs of endocervical involvement, and specimen collection.
Cervicitis is a clinical syndrome defined by the presence of cervical discharge, easy bleeding, or a yellow-greenish discoloration on a swab after insertion into the endocervix. Cervicitis can be caused by N gonorrhoeae and several other STDs (see Chapter 10).
Local infection of the endocervix ascends into the uterus, fallopian tubes, or adjacent structures in about 15% of women. Left untreated, this can result in upper reproductive tract infection or pelvic inflammatory disease (PID). Factors that may contribute to upper tract infections include menstruation, newly inserted intrauterine device, douching, adolescence, or previous PID. Clinical signs and symptoms of upper reproductive tract infection with gonorrhea are similar to those found with other intra-abdominal processes. None of the clinical signs and symptoms has both high sensitivity and specificity for PID, and the diagnosis is usually made on clinical grounds.
Pregnancy does not change the clinical presentation of gonorrhea in women. Pregnant women in the second and third trimester have demonstrated a lower incidence of PID. Whether this may represent a “barrier” effect of the products of conception or a change in sexual behaviors as the pregnancy progresses is unclear. Indeed, pregnant women have slightly higher rates of gonococcal pharyngitis that could be a marker for changing sexual behavior. Asymptomatic women may transmit infection throughout their pregnancy and postpartum periods to their sex partners.
Among women with endocervical infection, 10–20% also have pharyngeal infection likely associated with oral sexual exposure. Over 90% of pharyngeal infections are asymptomatic, but symptomatic disease includes an exudative pharyngitis or tonsillitis. Pharyngeal infection clears spontaneously in nearly all cases within 12 weeks of infection. All currently recommended treatment regimens are less efficacious against pharyngeal gonorrhea compared with uncomplicated genitourinary gonorrhea.
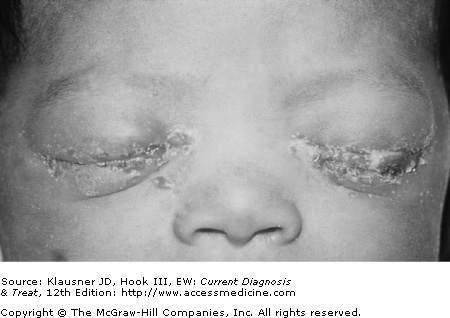
Peripartum transmission to the newborn may result in infection of the conjunctiva, rectum, or respiratory tract (see Figure 16–2). Genital or pharyngeal infection in young children is almost always an indication of sexual abuse.
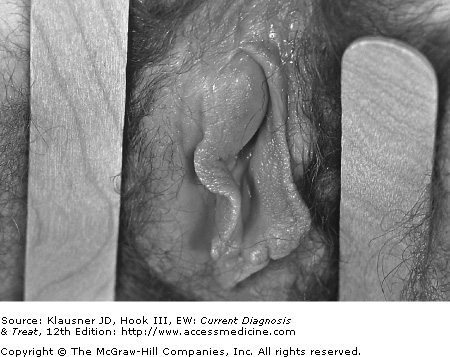
The anorectal area may be the only site of infection in 5% of women. Symptoms are rare but when they occur are similar to those experienced by MSM (pruritus and painless discharge). Another less common presentation of gonorrhea in women is infection of the Bartholin gland duct, which produces local erythema and pus at the posterior third of the labia majora (see Figure 16–3).