Chapter 561 Goiter
561.1 Congenital Goiter
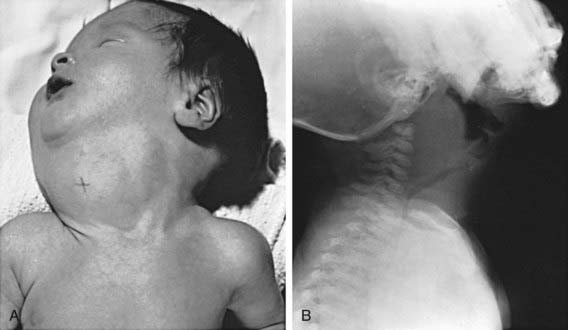
Enlargement of the thyroid at birth may occasionally be sufficient to cause respiratory distress that interferes with nursing and can even cause death. The head may be maintained in extreme hyperextension. When respiratory obstruction is severe, partial thyroidectomy rather than tracheostomy is indicated (Fig. 561-1).
Goiter is almost always present in the infant with neonatal Graves’ disease. These goiters usually are not large; the infant manifests clinical symptoms of hyperthyroidism. The mother often has a history of Graves disease, although discovery of neonatal hyperthyroidism can lead to the diagnosis of maternal Graves’ disease. Thyroid enlargement results from transplacental passage of maternal thyroid-stimulating immunoglobulin (Chapter 562.1). TSH receptor-activating mutations are also a recognized cause of congenital goiter and hyperthyroidism.
When no causative factor is identifiable, a defect in synthesis of thyroid hormone should be suspected. Neonatal screening programs find congenital hypothyroidism caused by such a defect in 1/30,000-50,000 live births. It is advisable to treat immediately with thyroid hormone and to postpone more-detailed studies for later in life. If a specific defect is suspected, genetic tests to identify a mutation may be undertaken (Chapter 559). Because these defects are transmitted by recessive genes, a precise diagnosis is helpful for genetic counseling. Monitoring subsequent pregnancies with ultrasonography can be useful in detecting fetal goiters (Chapter 90).
When the “goiter” is lobulated, asymmetric, firm, or large to an unusual degree, a teratoma within or in the vicinity of the thyroid must be considered in the differential diagnosis (Chapter 563).
Bizhanova A, Kopp P. Minireview: the sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinol. 2009;150:1084-1090.
Caron P, Moya CM, Malet D, et al. Compound heterozygous mutations in the thyroglobulin gene (1143delC and 6725G → A [R2223H]) resulting in fetal goitrous hypothyroidism. J Clin Endocrinol Metab. 2003;88:3546-3553.
Hashimoto H, Hashimoto K, Suehara N. Successful in utero treatment of fetal goitrous hypothyroidism: case report and review of the literature. Fetal Diagn Ther. 2006;21:360-365.
Koksal N, Akturk B, Saglan H, et al. Reference values for neonatal thyroid volumes in a moderately iodine-deficient area. J Endocrinol Invest. 2008;31:642-646.
Moreno JC, Klootwijk W, van Toor H, et al. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N Engl J Med. 2008;358:1811-1818.
Remuzzi G, Garattni S. Elimination of iodine-deficiency disorders in Tibet. Lancet. 2008;371:1980-1981.
Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2782-2893.
Zimmernamm MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372:1251-1262.