Gestational Trophoblastic Disease
Gestational trophoblastic disease comprises a spectrum of clinical and pathologic entities, which have in common the abnormal proliferation of trophoblastic tissue, the ability to locally persist or progress within the uterus, and the ability to invade adjacent tissues and metastasize. Also known as gestational trophoblastic neoplasias or gestational trophoblastic tumors, these diseases include benign complete and partial hydatidiform moles, which have uncertain malignant potential, as well as invasive molar pregnancies, and true neoplasms such as choriocarcinomas and placental site trophoblastic tumors. Described since the time of antiquity and previously often fatal, the revelation, in 1956, that metastatic choriocarcinoma could be successfully treated inaugurated an era when these diseases have become routinely curable, with gestational trophoblastic disease proving to be the standard against which the success of chemotherapy in treating other neoplasms has come to be judged. Nonetheless, improper diagnosis, delay in rendering care, selection of an inappropriate chemotherapy, or mismanagement of treatment-related complications can still lead to a poor outcome.
PROPERTIES OF NORMAL TROPHOBLAST
Trophoblastic cells are derived from the outer cell mass of the preimplantation embryo and have several unique properties. They affect the physical implantation of the embryo into the endometrium and produce human chorionic gonadotropin (HCG) in sufficient amounts to maintain early pregnancy. If the properties of normal trophoblast are considered, it is perhaps not surprising that abnormally proliferating trophoblast should possess the ability to behave in a clinically aggressive manner. Normal trophoblast, for example, demonstrates diminished expression of the major histocompatibility antigens and ABO antigens, which allows escape from maternal immunologic rejection (Bulmer and Johnson, 1985; Szulman, 1972). Normal trophoblast manifests the ability to invade tissue and grow into the maternal decidua, vessels, and myometrium. In addition, normal trophoblast has the ability to embolize into the maternal venous circulation and occasionally gain access to the systemic circulation (Covone and colleagues, 1984). Finally, the persistence of normal trophoblastic tissue in the myometrium, sometimes weeks or even months after parturition, implies that abnormal trophoblast can act in a similar manner.
HYDATIDIFORM MOLE
The most common entity in the spectrum of gestational trophoblastic disease is molar pregnancy, or hydatidiform mole, which, although not a true neoplasm, refers to pathologic conceptuses demonstrating varying degrees of proliferation of syncytiocytotrophoblastic tissue.
COMPLETE HYDATIDIFORM MOLE
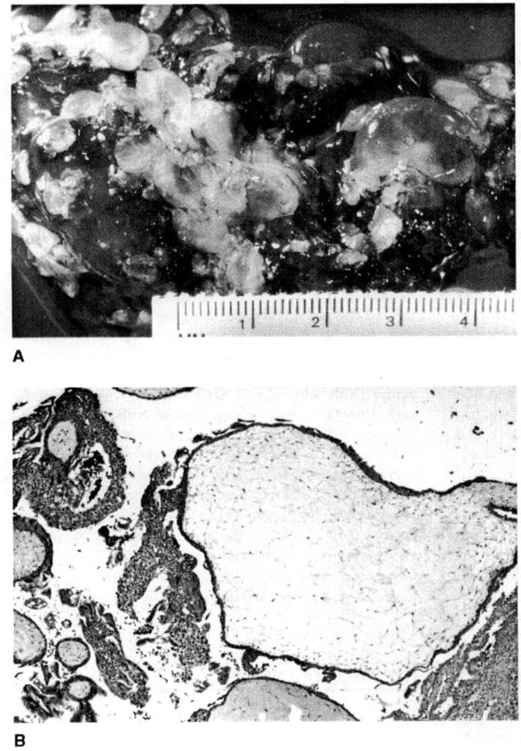
In a complete mole, there is diffuse hyperplasia of both cytotrophoblast and syncytiotrophoblast elements, with generalized edema of the chorionic villi and central cistern formation on a microscopic level yielding the gross appearance of “a bunch of grapes” (Fig. 34-1). Previous descriptions of histopathologic features suggested that complete moles lacked fetal blood vessels or embryonic tissues. These “classic” definitions of complete moles, however, may have been partly a reflection of the relatively advanced gestational age at which these entities were previously diagnosed (Newlands and colleagues, 1999). With many moles now evacuated at a much earlier gestational age, it is apparent that gross, or even microscopic hydropic, changes in the villi may be subtle or absent, and that fetal blood vessels may occasionally be present (Paradinas and colleagues, 1997).
FIGURE 34-1. A. Complete hydatidiform mole showing hydropic villi ranging in size from several millimeters to almost 2 cm. B. A large (>2 cm) hydropic, avascular villus with adjacent trophoblastic proliferation is seen in complete hydatidiform mole (X40). (Courtesy of Julianne S. Sandstad, MD.)
Cytogenetically, approximately 90–95 percent of complete moles have a 46 XX karyotype, and arise from diandric diploidy, or fertilization of an oocyte lacking a nucleus (“empty egg”) with a haploid 23X sperm, which subsequently reduplicates. Only 5–15 percent of complete moles arise as a result of dispermic fertilization of such an “empty egg,” which may result in a 46 XX or 46 XY karyotype. Complete moles with a 46 YY karyotype are not observed, as this is not compatible with life.
As is discussed later, the incidence of malignant postmolar sequelae after treatment of complete mole is significant, in the range of approximately 20 percent. The development of metastatic disease is not uncommon.
INVASIVE HYDATIDIFORM MOLE
Formerly known as chorioadenoma destruens, invasive mole is characterized by penetration of the uterine wall with proliferating trophoblast sharing the histologic features of a complete mole. Previously a diagnosis made at the time of hysterectomy, it is now less commonly seen due to the generally earlier diagnosis of trophoblastic disease. Evidence of myometrial invasion in molar pregnancy can sometimes be determined radiographically through the use of ultrasonography or MRI. This determination, however, is usually not pursued because the decision to administer chemotherapy after molar pregnancy evacuation will depend on HCG regression, and not on the presence of myometrial invasion, per se.
PARTIAL HYDATIDIFORM MOLE
As compared to complete moles, the degree of villous hydropic change and trophoblastic proliferation in partial moles is less pronounced. Fetal vessels and erythrocytes are typically present, and there may be macroscopic evidence of a fetus, although it is always nonviable. The histologic changes of partial mole may be subtle. In the absence of cytogenetic testing, the diagnosis is probably often not made, because triploidy accounts for 1–2 percent of clinically apparent spontaneous abortions (Jacobs and colleagues, 1982).
Cytogenetically, partial moles demonstrate diandric triploidy, with most possessing a 69 XXX or a 69 XXY karyotype, arising from dispermic fertilization of a haploid egg. 69 XXY partial moles are encountered very rarely, and 69 YYY conceptuses are not observed.
An important characteristic of partial moles, as compared to complete moles, is a far lower tendency to manifest malignant sequelae after uterine evacuation, estimated at perhaps 5 percent. The development of metastatic disease is unusual.
EPIDEMIOLOGY OF MOLAR PREGNANCY
Molar gestation has been estimated to occur with an incidence of slightly less than 1 per 1000 pregnancies (Palmer, 1994).
A prior history of molar pregnancy increases a patient’s chance of another mole in a subsequent pregnancy by 10-fold, and is the most influential risk factor for the disease. If a woman has had two prior molar pregnancies, her risk for a third is estimated to be 1 in 6.5 pregnancies (Bagshawe and colleagues, 1986).
The incidence of molar pregnancy appears to be increased in certain subgroups. Women at the extremes of reproductive age are at increased risk. Bagshawe estimated a 411-fold increased risk in women over the age of 50 and a six-fold increased risk for women under the age of 15 (Bagshawe and colleagues, 1986).
Geographical and ethnic differences in the rate of molar gestation have also been reported. Population-based studies have reported an approximately two-fold higher incidence of molar pregnancy in Japan and Saudi Arabia, as compared to North America (Palmer, 1994; Chattapodhyay and colleagues, 1988; Takeuchi, 1987). Low rates have been reported in the Netherlands and Paraguay (Franke, 1983; Rolon, 1990; and their colleagues).
Dietary factors, particularly deficiency in dietary fat and beta-carotene consumption, have been associated with increased risk of molar pregnancy in some studies (Berkowitz, 1985; Parazzini, 1988; and their colleagues). Interestingly, studies from Vietnam suggest that women living in areas where spraying with Agent Orange was common have a 13-fold increased risk of molar gestation (Constable and Hatch, 1985).
CLINICAL PRESENTATION AND DIAGNOSIS
It is evident that the clinical presentation of molar pregnancy is changing over time. Vaginal bleeding and passage of tissue in a patient with the finding of a uterus larger than dates is less common than previously, as routine obstetric ultrasound detects the disease far earlier in gestation than previously. Similarly, complications of hydatidiform mole associated with a large volume of trophoblast such as hyperthyroidism and theca lutein cysts, are caused by biologic cross-reaction of HCG with thyroid stimulating and gonadotrophin hormones, respectively. Hyperemesis and preeclampsia are less common than in previous experience.
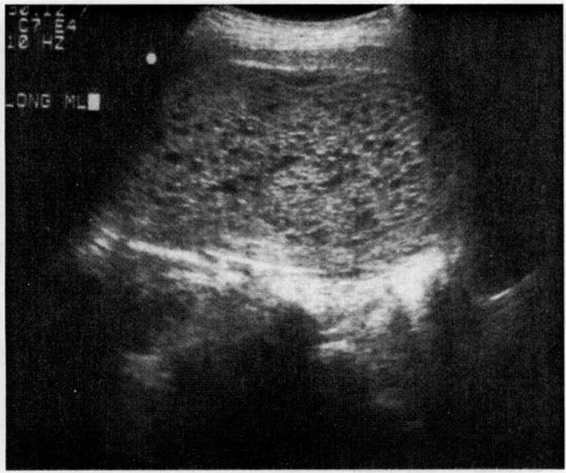
The modern diagnosis of molar gestation generally results from, or is confirmed by, pelvic ultrasonography (Fig. 34-2). While the classic “snowflake” pattern is frequently observed in the second trimester, the first trimester ultrasound diagnosis of molar gestation may be more problematic, as the vesicles are often too small to be visualized. In early pregnancy, a common diagnostic problem is the differentiation of a molar gestation from a missed abortion. Romero and colleagues (1985) reported, however, that an HCG level above 82,350 mlU/mL in the absence of fetal cardiac activity could correctly identify 88.8 percent of hydatidiform moles.
FIGURE 34-2. Ultrasound of hydatidiform mole. (Courtesy of Diane M. Twickler, MD.)
HUMAN CHORIONIC GONADOTROPHIN (HCG). The diagnosis and management of gestational trophoblastic disease is greatly facilitated by typically dramatic elevations in human chorionic gonadotrophin produced by the abnormally proliferating trophoblast that accurately reflect disease burden.
All patients with suspected molar pregnancy should undergo a baseline serum HCG evaluation. Conversely, molar pregnancy must be considered in the differential diagnosis of patients found to have a grossly elevated HCG level. In normal pregnancy, HCG rises exponentially until 10 weeks gestation, then decrease by 80 percent until the twentieth week of gestation, when they stabilize. In normal pregnancy, the ninety-fifth percentile maximal HCG is less than 100,000 mlU/mL whereas molar pregnancies may attain HCG levels easily in the several hundred thousand range.
Biochemically, HCG consists of an a and β subunit, non-covalently bound. The 92-amino-acid β chain has a common sequence homology with luteinizing hormone (LH), follicle stimulating hormone (FSH), and thyroid-stimulating hormone (TSH). The β subunit is specific for HCG. It is important to realize that multiple HCG-related molecules are present in the serum and urine of normal pregnancies and trophoblast disease, including degraded molecules, hyper-glycosylated molecules, free subunits, and fragments, but in varying proportions (Cole, 1998). “Nicked” HCG and free β subunit, in particular, is far more common in trophoblastic disease than in normal pregnancy. The original type of HCG immunoassay, the radioimmunoassay, used an HCG β sub-unit polyclonal antibody and measured all different forms of the β subunit (Cole, 1998). Modern quantitative HCG tests are sandwich-type immunoassays that use monoclonal antibodies with spectrometric, lanthanide, or luminescence detection systems, with varying specificities for detecting the various HCG fragments (Cole, 1998). It is important that a laboratory monitoring trophoblastic disease use an assay capable of detecting these various fragments.
PREOPERATIVE EVALUATION. In addition to a baseline HCG evaluation, patients with molar gestation should undergo complete physical examination, chest x-ray, a complete blood count, liver and renal function tests, and thyroid function tests.
SURGICAL TREATMENT
SUCTION CURETTAGE. Uterine evacuation through suction dilatation and curettage remains the mainstay of therapy for molar gestation. Given the potential for significant hemorrhage and uterine perforation, however, it should not be undertaken lightly. The procedure should be performed in a fully equipped and staffed operating room. Typed and cross-matched blood should be immediately available if needed. The attending anesthesiologist should be fully apprised of the potential for rapid blood loss and significant pulmonary compromise which can occasionally complicate the evacuation of bulky molar gestations. Large-bore peripheral IV lines should be in place prior to the initiation of the procedure, because of increased potential for rapid volume shifts or pulmonary complications in patients with moles greater than 16 weeks size. Some clinicians advocate placing indwelling central venous catheters prior to evacuation for invasive hemodynamic monitoring in these patients (Soper and colleagues, 1997). A laparotomy setup should be available in the rare circumstance that uterine perforation with the suction curette or intractable hemorrhage is encountered.
Except in rare circumstances, laminaria are not required, and, in fact, are discouraged prior to uterine evacuation, as they may stimulate uterine contractions and bleeding. In most cases, fetal parts are completely absent, allowing emptying of the uterus by means of a relatively small suction catheter (10-12 mm). Anesthesia is induced, the cervix is gently dilated, and the suction catheter inserted into the uterus. Pitocin infusion is started only after the cervix is dilated, and suction curettage is completed without delay. The quantity of blood and molar tissue evacuated from the uterus depends on gestational age, but can be truly impressive. Some authors have argued in favor of sharp curettage at the conclusion of suction, with that specimen submitted separately, in order to detect molar tissue in contact with myometrium, which might indicate invasive mole.
HYSTERECTOMY. In an occasional patient with molar pregnancy, consideration of primary hysterectomy as a therapeutic alternative to uterine evacuation may be appropriate. Older patients who clearly have completed childbearing, or those patients who are contemplating sterilization, particularly with molar pregnancies having a relatively high risk of postmolar sequelae, may choose to undergo primary hysterectomy. In one series, postmolar sequelae were reduced to just 3.5 percent after hysterectomy (Curry and colleagues, 1975). The ovaries are typically not involved in the disease process, and are removed only if other ovarian pathology exists or if advancing patient age has resulted in an informed consent discussion encompassing the pros and cons of prophylactic oophorectomy. While hysterectomy may be an option for some patients, evacuation by suction dilatation and curettage (D&C) remains preferable in most patients because of preservation of fertility and lower morbidity.
OTHER METHODS. Two other methods of evacuating molar gestation are of historical interest only and are mentioned only to strongly discourage their use. Labor induction with Pitocin or other oxytocic agents is hazardous and ineffective, and may encourage hemorrhage or embolization of trophoblastic material into the maternal circulation, sometimes with catastrophic consequences. Laparotomy with uterine hysterotomy has also been reported to increase the risk of postmolar malignant sequelae in addition to having increased morbidity versus D&C and has largely been abandoned (Curry and colleagues, 1975).
MANAGEMENT OF TWIN COEXISTENT MOLE AND INTRAUTERINE PREGNANCY
This rare phenomenon is estimated to occur at a frequency of 1 per 22,000 to 100,000 pregnancies, but may be more common in the era of assisted reproduction and ovulation induction (Stellar and colleagues, 1994).
The differential diagnosis of an obstetric ultrasound finding of an abnormal placental mass and a coexisting fetus includes a twin normal intrauterine pregnancy with complete mole, partial molar pregnancy, and nonmolar placental abnormality.
Steller reviewed 22 cases of multiple pregnancies with molar pregnancy and coexisting fetuses (Stellar and colleagues, 1994). Twenty-three percent of these gestations were the result of ovulation induction. Diagnosis was relatively late, with a mean estimated gestational age of 20.1 weeks, a markedly enlarged uterine size in most patients, and a mean preevacuation HCG titer of 839,000. Consequently, vaginal bleeding, toxemia, theca lutein cysts, and hyperemesis were common in these patients. Ultrasound correctly made the diagnosis of mole and coexisting twin in 68 percent of cases.
In Steller’s series, most patients with mole and coexisting fetus underwent immediate uterine evacuation via vaginal delivery, suction curettage, or hysterectomy. Several patients, however, were managed conservatively for up to 16 weeks in attempt to gain fetal viability. Often, this was not possible because of the appearance of severe pregnancy-induced hypertension and other complications. Five fetuses (23 percent) survived.
One notable feature of mole and coexisting fetus conceptions is a high incidence of postmolar malignant gestational trophoblastic disease, with 55 percent of patients in Steller’s report requiring chemotherapy.
The distinction between partial molar pregnancy and a complete mole with coexisting twin may be significant, because the former is almost always a nonviable triploid conceptus, and the latter a diploid conception which, as discussed above, sometimes may be carried to viability. Amniocentesis for karyotype analysis and molecular polymorphism analysis may be very helpful in making the clinical distinction (Ishii and colleagues, 1998).
SURVEILLANCE
Patients who have undergone molar pregnancy evacuation should undergo weekly HCG monitoring until normal levels are achieved, then monthly monitoring until 6–12 months of normal values have been achieved. Pelvic examination is performed every 2–4 weeks until bleeding has stopped and the HCG titer is under 1000.
CONTRACEPTION
Proper contraceptive counseling is essential following evacuation of molar pregnancy. Six months of normal levels after the first normal value has been proposed as a minimum duration, as recurrence risk will rapidly diminish after this time interval. Many clinicians are more comfortable recommending 1 year of contraception following the first normal HCG value, though these decisions are frequently tempered by maternal age considerations. Contraception will prevent the confusion and consternation wrought by a rising HCG value due to an intercurrent pregnancy. While metastatic disease will seldom manifest at HCG levels below the current threshold for the detection of intrauterine pregnancy by transvaginal ultrasonography, it may nonetheless be difficult for the clinician to attempt to wait out the rise in the HCG titer until the discriminatory zone of trans vaginal ultrasound (generally 1500 mlU/mL) is reached. The consequences of inadvertently treating an early intrauterine gestation with methotrexate, the cytotoxic of choice for nonmetastatic trophoblastic disease (see later), are severe, as the folate analogues are, in many cases, feticidal, and amongst the most teratogenic substances known; thus, they are strictly contraindicated in pregnancy. Aside from the concern of confusing an early intrauterine gestation with a recurrence of trophoblastic disease, it should be remembered that, occasionally, a second viable intrauterine pregnancy has followed closely on the heels of a molar pregnancy evacuation, actually preceding detection of the trophoblastic disease persistence or recurrence, thus complicating treatment immensely.
Oral contraceptive pills have remained the mainstay of contraceptive management following molar pregnancy evacuation. While modern low-dose oral contraceptives less reliably suppress gonadotrophins than older preparations, the suppression of FSH and LH have traditionally proven useful in preventing cross-reaction with assays designed to detect very low levels of HCG.
Initial concerns over a possible association between trophoblastic disease recurrence and oral contraceptive use were largely dispelled by a study by the Gynecologic Oncology Group, published in 1989. In this trial, 266 patients who had undergone molar pregnancy evacuation were randomized to oral contraception versus barrier contraception (Curry and colleagues, 1989b). No increase in recurrence risk was observed. Furthermore, twice as many patients in the barrier group became pregnant in the immediate fol-lowup period.
In patients for whom oral contraceptives are contraindicated, treatment with depo-medroxyprogesterone acetate is reasonable, though gonadotrophin suppression is less reliable. Patients who have completed childbearing may be candidates for surgical sterilization.
SUBSEQUENT REPRODUCTIVE OUTCOME
In a patient treated for a molar gestation, the risk of a second mole in a subsequent pregnancy is approximately 1 percent. In 1234 later conceptions occurring in patients treated for complete mole at the New England trophoblastic disease center, Berkowitz et al reported a 4 percent rate of major and minor congenital anomalies, with no particular anomaly noted, an experience comparable to that of the general population (Berkowitz and colleagues, 1998).
Because of these considerations, patients who have been treated for molar pregnancy should, in subsequent pregnancies, undergo early obstetric ultrasound to confirm a normal intrauterine pregnancy. Moreover, products of conception or the placenta from future pregnancies should be submitted for histopathologic review, and a quantitative HCG performed at 6 weeks postdelivery to confirm a normal value.
MALIGNANT GESTATIONAL TROPHOBLASTIC DISEASE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree