Essentials of Diagnosis
- • Clinical diagnosis is difficult because most genital chlamydial infections are asymptomatic and even when symptoms or signs are present, they are nonspecific.
- • Diagnosis relies on tests that detect the causative organism, Chlamydia trachomatis.
- • Nucleic acid amplification tests (NAATs) have the greatest sensitivity and can be performed on noninvasively collected specimens (eg, urine or self-collected vaginal swabs).
General Considerations
Chlamydia trachomatis is responsible for a wide spectrum of clinical disease, particularly in the genital tract (see Table 13–1). Despite the availability of effective antimicrobial therapy and improved preventive efforts, genital chlamydial infections remain a worldwide public health concern, and the World Health Organization estimates that 90 million new cases occur worldwide each year. Genital chlamydial infection remains the most commonly reported bacterial sexually transmitted disease (STD) in the United States, producing an estimated four million new infections each year, according to the Centers for Disease Control and Prevention (CDC).
| Urethritis (men or women) |
| Epididymitis |
| Prostatitisa |
| Cervicitis |
| Pelvic inflammatory disease |
| Endometritis |
| Salpingitis |
| Perihepatitis (ie, Fitz-Hugh-Curtis syndrome) |
| Bartholinitis |
| Proctitis and proctocolitis (men or women) |
| Conjunctivitis (men, women, or neonates) |
| Pharyngitis (men, women, or neonates) |
| Respiratory tract infection (neonates or immunosuppressed adults) |
| Upper: Rhinitis or bronchitis |
| Lower: Pneumonitis or pneumonia |
| Reiter syndrome (men or women) |
| Culture-negative endocarditis (men or women)a |
From the time genital chlamydial infections first became a reportable disease in the United States in 1986, a greater number of cases have been reported in women versus men, a finding that has been attributed to emphasis on chlamydial screening in women. Chlamydia causes significant morbidity, especially in women, who can develop upper genital tract infection (pelvic inflammatory disease [PID]), which can lead to chronic pelvic pain, tubal abscesses, ectopic pregnancy, and infertility; chlamydia is the leading preventable cause of infertility worldwide. Genital chlamydia can also increase the risk of acquisition and transmission of HIV.
Among the many risk factors for genital chlamydial infection (see Table 13–2), age is the strongest risk factor, with CDC surveillance studies demonstrating the highest chlamydial prevalence occurring in men and women younger than 25 years of age. A history of prior chlamydial infection is another strong predictor for current chlamydial infection. The majority of chlamydial infections in men and women are asymptomatic; therefore, the diagnosis of infection relies on identification of the organism through diagnostic testing. The availability of highly sensitive nucleic acid amplification tests (NAATs) should help to facilitate both improved rates of diagnosis and more widespread chlamydial screening, because such tests can be performed on noninvasively collected specimens (eg, urine and self-collected vaginal swabs). However, many barriers to screening exist, including lack of patient access to health care providers and lack of routine chlamydial testing in many medical settings.
| Adolescence and young adulthood |
| History of prior genital chlamydial infection |
| New or multiple sex partners |
| Nonwhite race or ethnicity |
| Lower socioeconomic status |
| Bacterial vaginosis |
| Oral contraceptive use (cervical ectopy) |
Current management of genital chlamydial infection relies on effective antimicrobial therapy for infected patients and their sex partners, and routine rescreening 3 months after treatment. Although antimicrobial resistance in chlamydial infections has not been a major concern to date, evidence is building that suggests this may be a future problem. Recurrence of chlamydial infection in women is common, and the CDC recommends retesting at approximately 3 months following therapy. Development of an effective chlamydial vaccine is one of the priorities for the prevention and control of chlamydia, but efforts to date have been hindered by an incomplete understanding of the human host immune response to C trachomatis.
Pathogenesis
Chlamydiae are obligate intracellular bacteria that are energy parasites of infected host cells. In the genital tract, C trachomatis primarily infects columnar and transitional epithelial cells but not squamous epithelial cells, explaining in part why chlamydial infection in the female genital tract occurs in the endocervix and upper genital tract, but not the vagina. C trachomatis can be classified through molecular typing into strains causing ocular infections (trachoma), strains causing lymphogranuloma venereum (LGV) infection, and strains causing the characteristic lower genital tract and other mucosal infections outlined in Table 13–1.
All chlamydiae undergo a unique intracellular developmental cycle (see Figure 13–1) that is completed in 48–72 hours when infected cells release newly replicated chlamydial organisms; as a result, the incubation time in genital chlamydial infection ranges from 7 to 21 days (compared with as few as 24–48 hours in gonococcal infection). Diagnostic assays, especially those other than NAATs, that are performed within the first few days after exposure to chlamydia may yield a false-negative result due to low organism burden at that point.
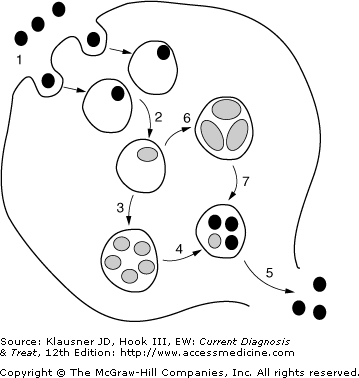
Figure 13–1.
The unique intracellular developmental cycle of Chlamydia trachomatis. The organism exists primarily in two forms: an elementary body (EB; black circles), which is the infective form that is metabolically inactive, and a reticulate body (RB, light-colored ovals), which is the reproductive form that is metabolically active. The numbered stages in the figure correspond to the following events of the cycle: (1) Endocytosis of the EB into an inclusion; (2) differentiation of the EB into an RB; (3) replication (several rounds) of the RB by binary fission; (4) redifferentiation of RBs into EBs; (5) exocytosis of EBs, which can go on to infect other cells; (6) under environmental stress (eg, immune mediators, nutrient deprivation, etc), RBs can differentiate into larger aberrant forms that are noninfectious and nonreplicating (ie, persistence); (7) upon removal of environmental stress, there is redifferentiation of aberrant forms into EBs.
The natural history of chlamydial infection is not yet fully understood, but Geisler and others have recently found that approximately 20% of individuals with genital chlamydial infection detected by screening have spontaneous resolution of their infection prior to returning for therapy, suggesting the host immune response, at least under some circumstances, eradicated the organism from the lower genital tract. In the majority of individuals, if not treated, infection tends to persist for weeks to months, and perhaps over a year in a small proportion of individuals.
Clinical Findings
Symptoms, physical examination findings, and immediate laboratory findings (eg, results of urethral or endocervical Gram stains) in lower genital tract chlamydial infection are nonspecific and do not reliably distinguish chlamydia from other infections causing the same genital tract syndromes. Diagnostic assays to detect C trachomatis are essential to confirm the diagnosis of chlamydia. Imaging studies are not usually needed in uncomplicated genital chlamydial infection and will not be discussed further.
Clinical findings in upper genital tract chlamydial infection (see Chapter 8) and anorectal infection (see Chapter 9) in men and women are discussed elsewhere in this book. Conjunctivitis caused by C trachomatis, usually resulting from autoinoculation from the genital tract, is rare. C trachomatis has been detected in the pharynx by culture and NAAT, yet it remains unclear whether clinically evident pharyngitis occurs and whether the organism is transmissible from the pharynx. The discussion that follows focuses on clinical findings in chlamydial infection of the lower genital tract.
Over 50% of men with urethral chlamydial infection are asymptomatic. Symptoms of chlamydial infection may include painful urination, urinary frequency, meatal itching or discomfort, and urethral discharge. If the patient has not voided recently, a urethral discharge may be apparent on examination and typically is clear or cloudy, often with mucus strands; accompanying the discharge may be meatal erythema or swelling, or both. However, it is important to recognize that asymptomatic urethral chlamydial infection without discharge occurs commonly. If no spontaneous discharge is noted, the urethra should be stripped (“milked”) from the base of the penis to the urethral meatus and examined again. Unlike the LGV chlamydial strains, the non-LGV strains do not cause genital ulcers or significant lymph node swelling.
At least 75% of women with endocervical chlamydial infection are asymptomatic, and even when symptoms are present, they are nonspecific and often overlap with symptoms found in other vaginal or endocervical infections. Symptoms may include new or increased vaginal discharge, intermenstrual bleeding, lower abdominal pain, or pain during intercourse. Up to 50% or more of women with endocervical chlamydial infection have concomitant urethral infection and may present with painful urination or urinary frequency, or both. Many women also have asymptomatic rectal infection. Symptoms and signs of proctitis are rare with such non-LGV chlamydial strains.
Even in the absence of symptoms, older studies using chlamydia culture have demonstrated up to one third of chlamydia-infected women have signs of infection on pelvic examination (Geisler and colleagues, utilizing NAATs, found fewer than 10% of asymptomatic women had signs of infection.) Mucopurulent endocervical discharge, easily induced endocervical bleeding (“friability”), and edematous ectopy are the signs most suggestive of chlamydial infection, yet all are nonspecific and may be seen in other sexually transmitted endocervical infections. Abnormal vaginal discharge (originating from the endocervix) may also be present.
Although cervical ectopy may predispose to chlamydial infection (through increased exposure to susceptible columnar epithelial cells), ectopy without edema or congestion may be present in up to 60–80% of sexually active female adolescents and young adults, especially those using oral contraceptives, and is not indicative of chlamydial infection.
Concomitant chlamydial infection of the Bartholin ducts may occur, manifesting as ductal erythema and swelling, often with purulent ductal exudate.
In chlamydial urethral infection, a Gram-stained smear made from an endourethral swab specimen often indicates the presence of urethritis (≥5 polymorphonuclear leukocytes [PMNs] per high-power field). However, Geisler and colleagues found that almost 20% of patients with chlamydial urethritis detected by NAAT had fewer than 5 or no PMNs visualized on a Gram-stained endourethral smear, suggesting that chlamydial infections may often mount a minimal inflammatory response or it may attenuate over time. Although an endourethral Gram stain can establish with a high degree of certainty whether gonorrhea is present in symptomatic urethritis, it does not exclude chlamydial infection, because C trachomatis cannot be visualized on a Gram stain.
If microscopy is not available, then urinalysis can be performed on a first-voided urine specimen (ie, the first 10–15 mL of urine voided), and in younger men, the finding of a positive leukocyte esterase test or microscopic pyuria (>10 white blood cells in spun urine sediment per high-power field) is consistent with urethritis due C trachomatis or another sexually transmitted pathogen. A positive leukocyte esterase test or pyuria in older men might represent a urinary tract infection or sexually transmitted urethritis, and diagnostic testing should address both.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree