Fluid and Electrolyte Management
Edward F. Bell
William Oh
Infants who are born prematurely or who are critically ill cannot regulate their own intake of fluids and nutrients. Moreover, enteral feeding is often limited by feeding intolerance or medical problems that preclude or limit use of the gastrointestinal tract for feeding. In other cases, the infant presents with disordered fluid and electrolyte balance as a primary result of an underlying illness. In all of these situations, water and electrolytes must be provided by prescription of the health provider. Prescribing the correct amounts of water and electrolytes helps to assure the infant’s healthy recovery.
The goal of fluid and electrolyte management is to replace losses of water and electrolytes so as to maintain normal balance of these essential substances during growth and recovery from disease. A subsidiary aim in the first days of life is to allow successful transition from the aquatic environment of the uterus into the arid extrauterine milieu. The principles of fluid and electrolyte management in the neonatal period are similar to those established for older children, except for some variations and specific features of body composition, insensible water loss (IWL), renal function, and neuroendocrine control of fluid and electrolyte balance.
To manage fluid therapy of newborns appropriately, the clinician should understand the normal physiologic mechanisms that govern water and electrolyte balance and the variations in these mechanisms that can occur in sick or premature infants. The clinician should develop a systematic approach to the estimation of fluid and electrolyte requirements for correction of deficits and replacement of ongoing losses, both normal and abnormal. Finally, the results of fluid and electrolyte management must be carefully monitored so that the intakes of water and electrolytes can be adjusted as needed.
BODY COMPOSITION OF THE FETUS AND NEWBORN INFANT
Changes in Body Water During Growth
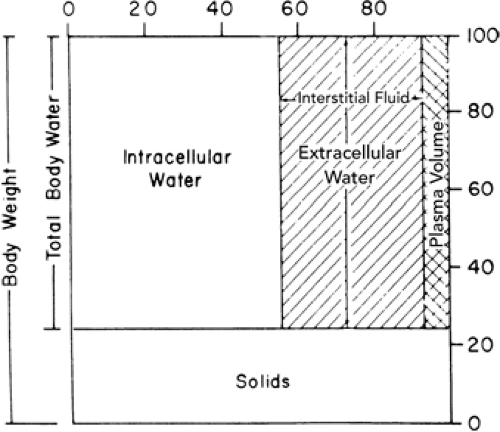
The total body water (TBW) is divided into two major compartments, intracellular (ICW) and extracellular (ECW). The ECW is further divided into the interstitial water and the plasma volume, which is the intravascular component of the ECW (Fig. 21-1).
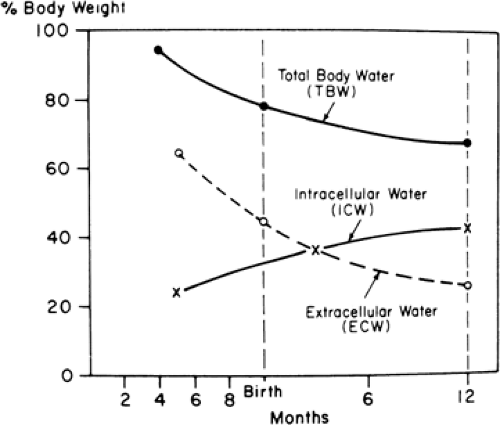
In the early stages of fetal development, a large part of the body consists of water (1). It has been estimated that TBW is 94% of the body weight during the third month of fetal life. As gestation progresses, the TBW per kilogram declines. By 24 weeks the TBW is approximately 86%, and by term it is about 78% of body weight (Fig. 21-2). There also are characteristic changes in the partition of body water between ECW and ICW during development. ECW decreases from 59% of body weight at 24 weeks of gestation to about 44% at term, and ICW increases from 27% to 34% of body weight during the same period (Table 21-1) (1,2,3,4,5,6). Infants born prematurely thus have higher TBW and ECW per kilogram than their term counterparts (7,8,9), and small-for-gestational-age infants have higher TBW per kilogram than do appropriate-for-gestational-age infants (9).
After birth, TBW per kilogram of body weight continues to fall, due primarily to contraction of the ECW (2,7,8,10,11,12,13). This mobilization of extracellular fluid occurs in conjunction with the improvement in renal function that takes place following birth (14,15), which is thought to occur as a result of increasing glomerular filtration rate and perhaps, too, as a result of increasing levels of the epithelial transport proteins involved in renal tubular function (16). It has also been suggested that atrial natriuretic peptide
plays a role in the postnatal contraction of the ECW (13). Various studies have shown an increase, decrease, or no change in the ICW after birth. ICW probably increases roughly in proportion to body weight in the first weeks of postnatal life (2,7,8,17). Thereafter, ICW increases faster than body weight and exceeds ECW by 3 months of age (Fig. 21-2) (1,2). These postnatal changes in body water and its partition between ECW and ICW are influenced by the intake of water and electrolytes (11,18). Failure to allow the normal postnatal contraction of ECW in premature infants may increase the risk of significant patent ductus arteriosus (PDA) (19), necrotizing enterocolitis (NEC) (20,21,22), and bronchopulmonary dysplasia (BPD) (23,24).
plays a role in the postnatal contraction of the ECW (13). Various studies have shown an increase, decrease, or no change in the ICW after birth. ICW probably increases roughly in proportion to body weight in the first weeks of postnatal life (2,7,8,17). Thereafter, ICW increases faster than body weight and exceeds ECW by 3 months of age (Fig. 21-2) (1,2). These postnatal changes in body water and its partition between ECW and ICW are influenced by the intake of water and electrolytes (11,18). Failure to allow the normal postnatal contraction of ECW in premature infants may increase the risk of significant patent ductus arteriosus (PDA) (19), necrotizing enterocolitis (NEC) (20,21,22), and bronchopulmonary dysplasia (BPD) (23,24).
Solute Distribution in Body Fluids
The major cation in the blood plasma is sodium (Fig. 21-3). Potassium, calcium, and magnesium constitute the balance of the cation fraction. The primary anion is chloride, with protein, bicarbonate, and some undetermined anions constituting the balance of the anions. The interstitial fluid (i.e., nonplasma ECW) has a solute composition that is similar to plasma except that its protein content is lower. The ICW contains potassium and magnesium as its primary cations, and phosphate, both organic and inorganic, is the major anion, with bicarbonate contributing a smaller fraction.
TABLE 21-1 CHANGES IN BODY WATER AND ELECTROLYTE COMPOSITION DURING INTRAUTERINE AND EARLY POSTNATAL LIFE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The electrolyte composition of the body fluids of the newborn infant is largely determined by gestational age. Premature infants contain more sodium and chloride per kilogram of body weight than term infants (3,4,5) because of their larger ECW (Table 21-1). Total body potassium content largely reflects ICW and is similar or slightly lower
per kilogram of body weight in premature infants than at term (3,6). These concepts are important in the management of fluid and electrolyte therapy for newborn infants.
per kilogram of body weight in premature infants than at term (3,6). These concepts are important in the management of fluid and electrolyte therapy for newborn infants.
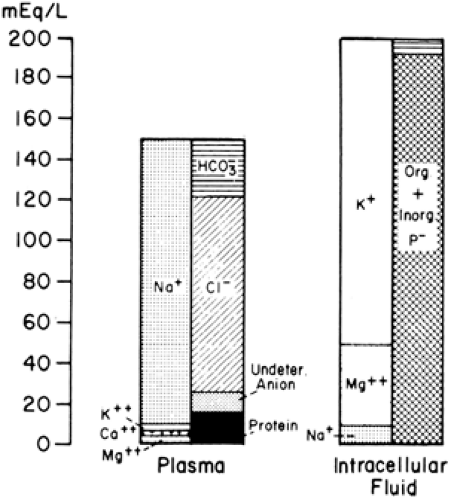 Figure 21-3 Ion distribution in the blood plasma, which represents extracellular fluid, and in the intracellular fluid compartment. |
INSENSIBLE WATER LOSS
The loss of water by evaporation from the skin and respiratory tract is known as insensible water loss (IWL). About 30% of IWL normally occurs through the respiratory tract as moisture in expired gas (29,30,31), with the remaining 70% lost through the skin. IWL can be expressed in reference to body surface area (m2) or weight (kg). IWL depends more on surface area than weight, but it is commonly expressed per kilogram because weight is more easily determined than area.
TABLE 21-2 FACTORS AFFECTING INSENSIBLE WATER LOSS IN NEWBORN INFANTS | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
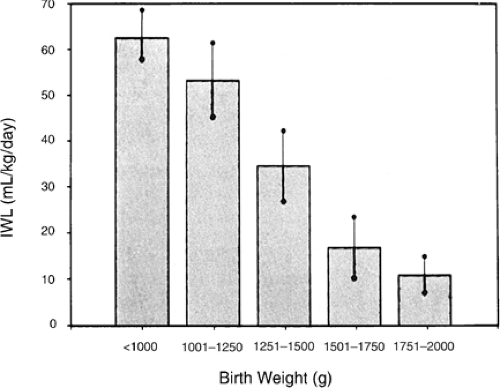
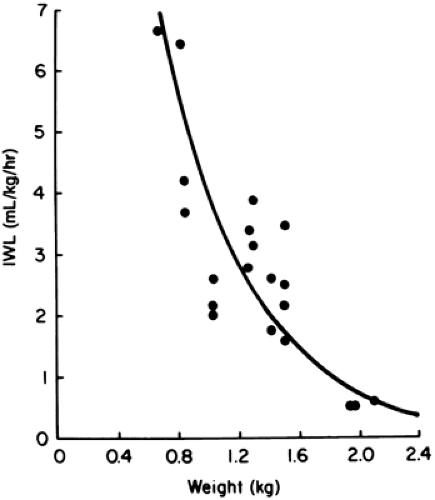
A number of factors are known to influence IWL in a predictable manner (Table 21-2) (29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60). When expressed per kilogram of body weight, IWL is inversely proportional to birth weight and gestational age (Figs. 21-4 and 21-5) (32,33,35). In other words, smaller, more immature infants have larger IWL per kilogram (Table 21-3). The
same is true if IWL is expressed per square meter of body surface (36,37). Therefore, although the greater IWL of smaller premature infants is partly due to the increased ratio of surface area (skin and respiratory tract) to body weight, it also is thought to be related to their thinner skin, greater skin blood flow, larger body water per kilogram of body weight, and higher respiratory rate. Because skin permeability to water varies inversely with gestational age, the degree of immaturity is an important determinant of cutaneous IWL independent of birth weight.
same is true if IWL is expressed per square meter of body surface (36,37). Therefore, although the greater IWL of smaller premature infants is partly due to the increased ratio of surface area (skin and respiratory tract) to body weight, it also is thought to be related to their thinner skin, greater skin blood flow, larger body water per kilogram of body weight, and higher respiratory rate. Because skin permeability to water varies inversely with gestational age, the degree of immaturity is an important determinant of cutaneous IWL independent of birth weight.
Factors That Increase Insensible Water Loss
An increase in minute ventilation increases the respiratory IWL (38) as long as the water vapor pressure is less in the inspired than in the expired gas. Increased minute ventilation may occur in infants with cardiac disease, pulmonary dysfunction, or metabolic acidosis.
TABLE 21-3 AVERAGE INSENSIBLE WATER LOSSa OF PREMATURE INFANTS IN INCUBATORS | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Environmental temperature above the neutral thermal zone increases IWL in proportion to the increment in temperature (29,39,40). This effect can occur even without a rise in body temperature. In contrast, a subneutral environmental temperature is not associated with reduced IWL, although metabolic heat production is increased (40). Increased body temperature, whether caused by fever or environmental overheating, elevates IWL (29,39).
Skin breakdown or injury disrupts the barrier against cutaneous evaporation and raises IWL. Skin trauma from thermal, chemical, or mechanical injury is common among critically ill, small, premature infants. Such injury may result from removal of tape and adherent monitoring devices or from prolonged skin exposure to disinfectant solutions. IWL also is increased in conjunction with the skin manifestations of essential fatty acid deficiency, a potential problem in infants receiving fat-free parenteral nutrition. Congenital skin defects, such as those seen in gastroschisis, omphalocele, and neural tube defects, are associated with increased IWL until surgically corrected.
Use of nonionizing radiant energy, in the form of either a radiant warmer or phototherapy, has been shown to increase IWL by about 50% (33,41,42,43,44,45,46,47,48). For infants in incubators with controlled air temperature, the increase in IWL with overhead phototherapy is most likely a result of increased body temperature because of the warmer incubator walls (46). For infants in incubators operated to control skin temperature, the rise in IWL with phototherapy can be explained by the lower absolute humidity resulting from the reduced air temperature that accompanies the warming of the incubator walls by the phototherapy. The impact on IWL of phototherapy delivered by fiberoptic blankets or pads is not known but is probably negligible unless the blanket produces a warmer or moister microenvironment around the infant. Investigators using direct measurements of transepidermal and respiratory water loss have obtained
conflicting results regarding the effect of overhead phototherapy on IWL. One group (47) found an increase in transepidermal water loss with phototherapy, but another did not (61,62).
conflicting results regarding the effect of overhead phototherapy on IWL. One group (47) found an increase in transepidermal water loss with phototherapy, but another did not (61,62).
TABLE 21-4 RELATIVE AND ABSOLUTE HUMIDITY AS RELATED TO INSENSIBLE WATER LOSS IN INCUBATORS AND UNDER RADIANT WARMERS | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
If an infant’s IWL is measured at the same skin temperature under a radiant warmer and in an incubator, the IWL is higher (by about 50%) under the radiant warmer. IWL is higher because absolute humidity (water vapor pressure) is lower under the radiant warmer than in the incubator (44). This may be true even if relative humidity is higher under the radiant warmer (43,44) because the lower air temperature with the radiant warmer means that the saturation pressure of water vapor is considerably lower than in the incubator (Table 21-4). This finding has been confirmed using direct measurements of transepidermal water loss (45). It is now understood that the higher IWL with radiant warmers arises from the lower ambient water vapor pressure and not from higher air velocity or a direct effect of nonionizing radiation on the skin. The same phenomenon explains the effect of phototherapy on IWL of infants in incubators operated by skin temperature servocontrol. The effects on IWL of radiant warmers and phototherapy are additive; the IWL with the combination is approximately twice as large as in an incubator without phototherapy (43,48).
Factors That Reduce Insensible Water Loss
Increasing the humidity or water vapor pressure of inspired gas reduces respiratory IWL. The inspired humidity is raised by humidifying the air-oxygen mixture delivered to a head hood or directly to the infant’s upper airway (e.g., via nasal cannula, face mask, or endotracheal tube) if respiratory support is required. If the temperature and water content of the inspired and expired gas are the same, the respiratory IWL will be entirely eliminated (51). Increasing ambient humidity, for example in an incubator, reduces total IWL, but respiratory IWL is decreased more than cutaneous IWL (29); a threefold increase in ambient water vapor pressure, from an average of 7 to 25 mm Hg, resulted in a 30% reduction in total IWL. Increasing ambient humidity is facilitated by the design of certain recent models of incubators. The use of incubator humidification systems should not be overlooked as a way of reducing IWL.
Plexiglas heat shields are effective in reducing the IWL of small premature infants in incubators (32,44), especially if the ends are at least partially enclosed to decrease air movement near the skin. Plexiglas heat shields are not effective for infants under radiant warmers (44,52) because Plexiglas is opaque to the infrared energy produced by the radiant heaters. Thin barriers of saran and other materials reduce IWL of infants under radiant warmers while allowing the infrared heat to reach the skin (52). These heat shields presumably reduce IWL by limiting air movement and raising water vapor pressure near the infant’s body surface.
Thin plastic blankets have been found to reduce IWL by 30% to 70% for infants under radiant warmers and in incubators (52,53,54). Chambers made of thin plastic material also reduce IWL by a similar amount (54,55). Semipermeable membranes (56,57,58) and waterproof topical agents (59,60) reduce IWL from the covered areas by an average of approximately 50%.
Knowledge of these factors that affect IWL is essential for estimating the water intake required by newborn infants and for making appropriate adjustments in water intake with changes in care. Of all infants, premature and critically ill infants are the ones whose IWL is most significantly influenced by these factors. This is especially true for the extremely premature infant, of whom more are surviving each year. However, these are exactly the infants for whom precise maintenance of fluid and electrolyte balance is most important and for whom the margin of error is smallest.
NEUROENDOCRINE CONTROL OF FLUID AND ELECTROLYTE BALANCE
The pituitary gland, adrenal cortex, parathyroid glands, and heart are the major organs producing hormones involved in the regulation of water and electrolyte balance in the body. The basic mechanisms by which the antidiuretic hormone, arginine vasopressin (AVP), is produced and secreted by the posterior pituitary gland appear to be intact in newborn infants (63,64,65), even in those who are born prematurely (66). It is not clear at what age precise quantitative hypothalamic control of AVP production is established. However, it is known that even in the first week of life, breast-fed term infants release AVP in response to a 10% loss of body weight (65).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree