Ultrasound picture showing needle guide centered on intrahepatic vein.
Intraperitoenal transfusions rely on absorption of the red cells into the fetal circulation via the subdiaphragmatic lymphatics and thoracic duct. The presence of ascites reduces the efficacy of this process, significantly reducing absorption and increasing mortality. The main advantage of the intraperitoneal route is that it can be performed at earlier gestations (in very high-risk situations)(<18 weeks) or when access to the fetal vasculature is hampered by fetal lie. The needle is inserted through the anterior abdominal wall, below the umbilical vein but above the fetal bladder. Correct placement of the needle intraperitoneally can be confirmed by aspirating ascetic fluid in the hydropic fetus or via observing an infusion of saline into the cavity.
Complications include the risks of an invasive procedure during pregnancy and a perinatal mortality rate of 1.6–2%[6,7] due to the specific complications of umbilical cord hematoma resulting in arterial (sometimes transient) vasospasm, fetal bradycardia or fetal exsanguination from needle puncture site, leading to fetal compromise and resultant emergency premature delivery (particularly in fetal alloimmune thrombocytopenia). There is a risk of fetomaternal hemorrhage with the resultant risk of increasing maternal antibody levels or even the development of new red cell antibodies.
Outcomes for pregnancies managed with intrauterine transfusions are good with an overall survival rate of 84%, higher survival is seen in pregnancies that are nonhydropic compared to hydropic (94% versus 74%)[7]. The study with the longest follow-up is the LOTUS study from Leiden in the Netherlands[8]. A total of 291 children who underwent intrauterine transfusion for hemolytic disease of the fetus were evaluated at a median age of 8.2 (range, 2–17) years. Cerebral palsy was detected in six (2.1%) children, severe developmental delay in nine (3.1%) children, and bilateral deafness in three (1.0%) children. The overall incidence of neurodevelopmental impairment was 4.8% (14/291). In a multivariate regression analysis including only preoperative risk factors, severe hydrops was independently associated with neurodevelopmental impairment (odds ratio (OR), 11.2; 95% confidence interval (CI), 1.7–92.7)[8].
Needle aspiration for drainage of fluid from body cavities
Under ultrasound guidance any fluid-filled cavity can be aspirated. This may be performed for two reasons: either to sample the fluid for diagnostic purposes (e.g., fetal urine in lower urinary tract obstruction) or to reduce the amount of fluid within an anatomic space to prevent dilatation or compression of the associated organ (e.g., pleural effusions) until delivery can occur. Performing this procedure for the latter indication should be carefully considered as it is likely that the fluid will re-accumulate and that multiple procedures will be required. In this instance, shunting of the cavity may also be considered. In some cases, drainage of a cavity may be required prior to delivery to either facilitate vaginal delivery, e.g., abdominal cysts (enlarging the abdominal circumference with risk of dystocia) or to facilitate neonatal resuscitation and management, e.g., pleural effusions to facilitate ventilation. Palliative procedures may also be performed to facilitate vaginal delivery, e.g., drainage of a hydrocephalus, which can be performed percutaneously prior to labor or vaginally intrapartum.
Ultrasound-guided shunt insertion
Thoracic
The procedure for thoracoamniotic shunting is the same as that for vesicoamniotic shunting (VAS) as are the complications. The “pigtail” catheter is inserted into the lower pleural cavity, posterior to the midaxillary line. The main indication for shunting is an effusion, likely to be an isolated chylothorax with no chromosomal anomalies, which rapidly accumulates after thoracocentesis. The rationale for treatment is to (a) reduce the risk of pulmonary hypoplasia and (b) reduce the risks of cardiovascular compromise (and the development of hydrops fetalis). A systematic review of pulmonary drainage (shunt, surgery or drainage), including 16 observational studies and 608 fetuses, concluded that pulmonary drainage did not improve perinatal survival in cystic lung lesions (mainly congenital cystic adenomatoid malformations and pulmonary sequestrations) compared with no drainage overall. However, there was a marked improvement with this therapy in a subgroup of fetuses with fetal hydrops fetalis (but not in the subgroup uncomplicated by fetal hydrops fetalis)[9]. Only two of the included studies looked at treatment for primary hydrothoraces and there was no significant improvement in survival with treatment; sub-group analysis according to the presence of hydrops fetalis was not possible in this group[9].
Urinary tract
Fetal VAS is the most common antenatal intervention for lower urinary tract obstruction (LUTO) and was first reported by Golbus et al in 1982[10]. The procedure is performed under ultrasound guidance with prophylactic antibiotics and maternal sedation if required. Amnioinfusion may also be required prior to shunt insertion if there is anhydramnios or severe oligohydramnios. The shunt is inserted via a trochar percutaneously and consists of a pigtail catheter (either Harrison or Rocket), with one end in the fetal bladder and the other draining into the amniotic cavity, thus bypassing the obstruction.
Data relating to the efficacy of VAS in the management of LUTO from cohort studies has been summarized in a systematic review that concluded that VAS compared to no treatment improved perinatal survival (OR 3.86; 95% CI 2.00–7.45) but that this effect was more significant in a group with poor prognosis on fetal urinalysis (OR 13.85; 95% CI, 1.25–153.05) (Figure 28.2). However, when subgroup analysis was performed to look at the effects on survival with normal renal function, there was a suggestion that VAS had an adverse effect (OR 0.50; 95% CI 0.13–1.90)[11] (Figure 28.3). Due to the biases in cohort data highlighted in the systematic appraisal of the evidence, there was a need for an randomized controlled trial (RCT) of VAS versus conservative management with long-term follow-up into childhood. The percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO) trial was a multicenter RCT with the objectives to determine if intrauterine VAS for fetal bladder outflow obstruction, compared with conservative care, improved prenatal and perinatal mortality and renal function for survivors[12]. Unfortunately, due to poor recruitment and the high numbers of parents that opted for termination of pregnancy (TOP) (46.9%), the PLUTO trial closed early to randomization in December 2010 with 31 babies in the randomized arm, 46 registered and 68 pregnancies that had had a TOP. Of those randomized to VAS, 8/16 (50%) survived to 28 days compared with 4/15 (27%) of those randomized to conservative management (relative risk (RR), 1.88; 95% CI, 0.71–4.96; P = 0.27). All 12 newborn deaths were as a result of pulmonary hypoplasia in the early neonatal period. One baby in each arm of treatment subsequently died prior to 1 year of life due to renal failure. RR estimates for survival at 1 and 2 years were similar to those seen at 28 days. The conclusion was that survival appeared higher in those fetuses receiving VAS, but uncertainty in the direction and magnitude of the effect remained. Overall long-term prognosis for all babies regardless of treatment received was poor, with only two babies surviving to 2 years of age with no renal impairment (Figure 28.4).
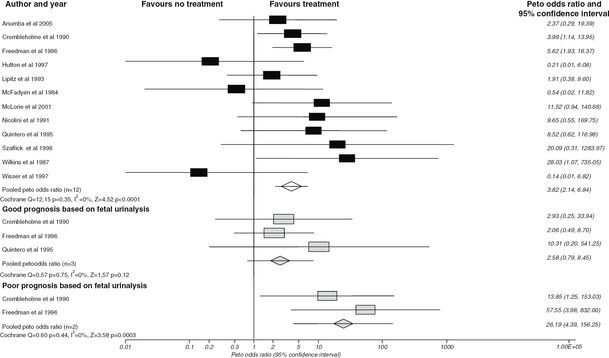
Effect of antenatal intervention on perinatal survival compared with no treatment (including voluntary termination of pregnancies) stratified by predicted prognosis.
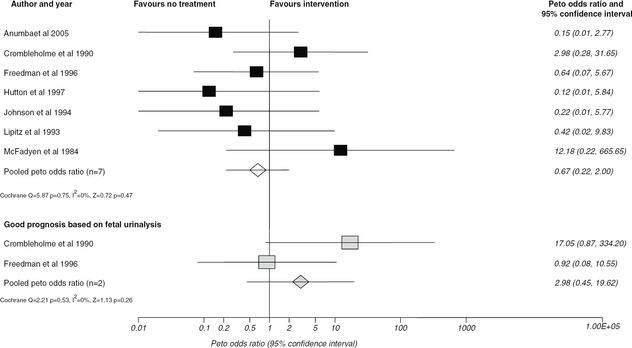
Effect of antenatal intervention compared to no treatment on postnatal survival with normal renal function (excluding intrauterine deaths and voluntary termination of pregnancy) stratified according to predicted prognosis.

Outcomes for babies within the PLUTO trial (extrapolated to 100 babies). VAS, vesicoamniotic shunting.
Cohort studies have reported outcomes for survivors past 5 years of age following VAS and shown that while these children have normal quality of life scores and normal cognitive ability, a significant proportion have difficulty voiding or incontinence and recurrent urinary tract infections. Up to a third will require dialysis and/or transplantation[13].
Thus, counseling of parents faced with this diagnosis for their baby must include a multidisciplinary approach involving fetal medicine specialists, neonatologists and pediatric urologists/nephrologists, recognizing that the prenatal counseling is complex and stressful for parents. Qualitative research has shown that parents often consider TOP as the long-term outcomes are morbid with significant consequences for the family unit[14].
Fetoscopy
Diagnostic and therapeutic cystoscopy
The limitations of VAS in terms of patient selection, diagnosis of underlying pathology and inability to correct the blockage, led to the development of fetal cystoscopy by Quintero et al. in 1995[15]. The proposed advantages of this technique are that it can be used for diagnosis of underlying pathology as well as treatment in LUTO. The most common technique is percutaneous anterograde fetal cystoscopy under maternal anesthesia (regional or local) with fetal anesthesia (fentanyl and pancuronium) under direct ultrasound guidance (Figure 28.5). A fetal urine sample can be obtained and then the bladder neck is inspected. If posterior urethral valves are identified, options for treatment and perforation of the valves are hydroablation, guide wire or laser fulguration. The finding of a nonmembrane like structure at the bladder neck suggests urethral atresia. A systematic review of the literature has assessed the effectiveness of fetal cystoscopy for diagnostic accuracy and effectiveness and demonstrated a high sensitivity (100%) and specificity (85.7%) for the correct diagnosis of LUTO[16]. However, comparing VAS to fetal cystoscopy, there appeared to be no significant improvement in perinatal survival OR 1.49 (95% CI, 0.13–16.97)(Figure 28.6). Further investigation in the form of a RCT is required to compare VAS to fetal cystoscopy[16] with long-term follow-up of babies to allow confirmation of diagnosis postnatally and long-term outcomes, such as renal function and bladder function, to be assessed. Complications of fetal cystoscopy are similar for any fetoscopic technique, with no reports of fetal bladder damage secondary to treatment in the small number of cases reported.
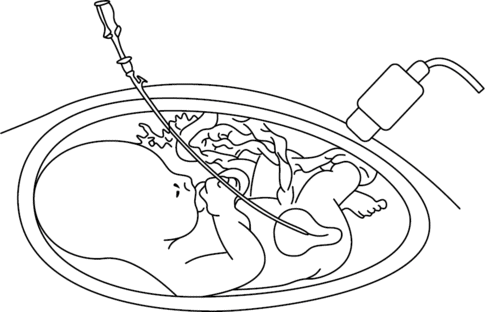
Technique for fetal cystoscopy.
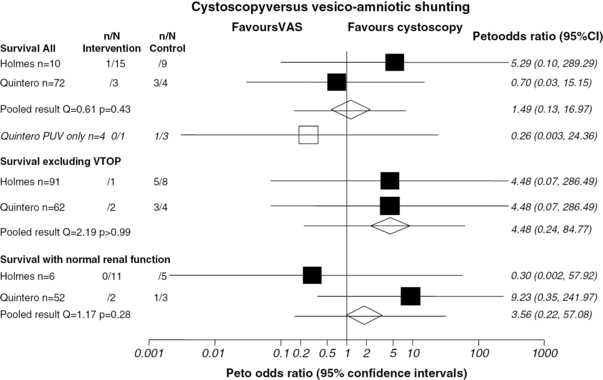
Effect of fetal cystoscopy on perinatal survival compared to vesicoamniotic (VAS) shunting (results in italics are sub-groups). VTOP, voluntary termination of pregnancy; PUV, posterior urethral valves.
The treatment of severe twin-to-twin transfusion syndrome: fetoscopic laser coagulation
Twin-to-twin transfusion syndrome (TTTS) is a condition of monochorionic (MC) pregnancies associated with a hemodynamic imbalance between twin fetal circulations. Untreated, the condition carries a mortality rate of 80–90%. The pathology and natural history of TTTS has been detailed in Chapter 7.1.
Historically, there were three main treatment options: selective feticide, serial amnioreduction and amniotic septostomy. Serial amnioreduction was the initial mainstay of treatment as it was easy to perform and helped improve maternal symptoms secondary to distension, and reduced the risk of preterm labor. Although an improvement in Doppler studies has been reported with the use of this treatment[17], due to an uncertain mechanism, it has variable survival rates from 37–60%[17,18], with 17–33% of survivors developing neurologic damage. In the 1990s, septostomy was introduced[19], again with the advantage of being a simple treatment, its mechanism being to equalize the pressure difference between the two sacs. Outcomes include survival rates of up to 83%[20] but long-term morbidity data is lacking. Both amnioreduction and septosotomy have the risk of rupture of membranes, intrauterine infection, placental abruption, miscarriage and preterm labor, and the latter iatrogenically converts the pregnancy to a monoamniotic one with the risk of twin and cord entanglement.
Fetoscopic laser coagulation (FLC) of placental vessels was introduced in the 1990s[21] and has been proven to be effective via RCT compared with amnioreduction[22]. It is the only treatment to address the underlying pathology by ablating the intertwin anastomoses along the chorionic plate.
A Cochrane review reported for treatment of TTTS with FLC: overall death, RR 0.87 (95% CI, 0.55–1.38); death of at least one infant per pregnancy, RR 0.91 (95% CI 0.75–1.09) and death of both infants, RR 0.67 (95% CI 0.27–2.10) when compared with amnioreduction. More babies were alive without any neurologic abnormality at the age of 6 years in the laser group than in the amnioreduction groups (RR 1.57; 95% CI 1.05–2.34 adjusted for clustering, one trial). A recent publication summarizing the outcomes for seven fetal medicine teams, with over 100 patients for each team, reported survival rates of 73–90.5% of at least one twin[23].
The treatment of stage 1 TTTS remains controversial. It can be managed with close observation, particularly if the cervical length is (reducing the risk of preterm delivery) and the condition remains stable[24]. Others recommend consideration of invasive treatment even at an early stage[22,25,26]. A cost-effective analysis, which looked at no treatment, amnioreduction and FLC, found the dominant strategy to be FLC using quality-adjusted life years (QUALYs – unit of assessment for clinical effectiveness as utilized by the National Institute for Health and Care Excellence) as an outcome measure. Amniodrainage did perform better than no treatment alone but was not as effective or cost-effective as FLC. This further supports the rationale for FLC being the first-line treatment for TTTS[25,27].
FLC is performed between 16 and 26 weeks. The technique involves either a regional or local anesthetic and a 3-mm trocar is inserted under ultrasound guidance into the recipient sac. Ideally, the trocar is inserted at right angles to the donor’s longitudinal axis to allow the operator to visualize the intertwin membrane and anastomoses. The trocar is used to introduce a fetoscope and laser fibre (using either a Nd:Yag or diode laser at 40–60 Watts) and anastomoses are coagulated under direct vision (Figure 28.7). In some cases, amnioinfusion is used to facilitate the procedure, with drainage at the end to a normal maximum pool depth. Techniques for identifying and selecting vessels to be coagulated involve: the selective sequential technique (SLCPV), avoiding the AA and VV anastomoses; the nonselective technique, coagulating all vessels crossing the membrane to ensure none are missed; and the “Solomon” technique. The latter involves completing an initial selective coagulation and then coagulating a dividing line across the vascular equator. A recent RCT comparing SLCPV to the Solomon technique reported a reduction in postoperative fetal morbidity in severe TTTS. There was a reduction in twin anemia polycythemia sequence (TAPS) (3% versus 16% for the standard treatment; OR 0.16, 95% CI 0.05–0.49) and less recurrence of TTTS (1% versus 7%; OR 0.21, 95% CI 0.04–0.98). Perinatal mortality and severe neonatal morbidity did not differ significantly between the two groups[28]. Tocolysis and prophylactic antibiotics are usually employed.
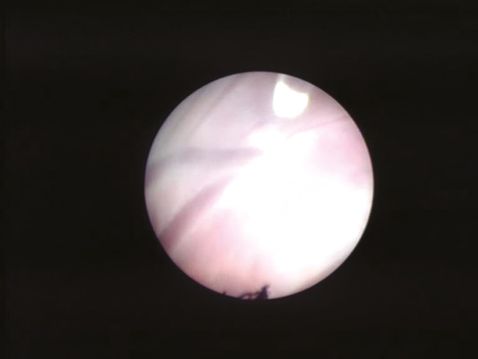
Fetoscopic visualization of an intertwin arteriovenous anastomosis. The vein is “cherry red” in color and the artery is “blue in hue.” The laser fiber tip (500 μm at 1 o’clock in the image) can be visualized along with a “white patch” between the artery and vein produced by coagulation at 40W.
Early referral to a tertiary center is important for assessment, and when FLC is being considered, centers need to demonstrate a high throughput of cases. Morris et al. demonstrated the significance of a learning curve, not only for the center but for the individual, and the effects of experience on outcome[23]. Maternal complications of this technique range from pain due to amniotic fluid leak into the peritoneal cavity, chorioamniontis, ruptured membranes and bleeding to severe complications of placental abruption and pulmonary embolus. Maternal complications are likely to be under-reported with rates of 5.4% overall and 1.8% for severe complications as stated above[29]. Fetal complications can occur in the first 6 days (early), including single and double intrauterine death and TAPS. Late complications include recurrent or reversed TTTS, late TAPS, intrauterine death (with risks to the surviving twin if single twin demise). Recurrent TTTS and TAPS are due to residual anastomoses or revascularization of ablated anastomoses and are found in up to 33% of placentas following FLC with SLCPV[30]. As discussed earlier, the Solomon technique confers a reduction in these late complications[28]. There is a high risk of preterm labor, with 30.5% of cases delivering before 32 weeks[31]. Thus, even after successful therapy it should be recognized that the pregnancy remains high risk and many centers advocate delivery between 32 and 3 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


