Fetal Therapy
Mark I. Evans
Mark P. Johnson
Alan W. Flake
Yuval Yaron
Michael R. Harrison
Over the past three decades, physicians from multiple specialties have developed numerous methods for the diagnosis of structural and physiological fetal abnormalities (1,2). When they are severe or lethal, pregnancy termination is viewed by many as a reasonable consideration. For couples in countries that permit its availability and in cultures in which the fetus does not have more rights than the mother, a variable portion of patients chose this option (3,4). With more moderate fetal anomalies, obstetrical care can be modified to optimize outcomes and prevent secondary complications. In some instances, prenatal treatments of the underlying problem have become possible. In general, structural malformations are more logically approached with surgery, although metabolic disorders may benefit from pharmacological or genetic therapies (2).
Fetal therapy has evolved into four major areas: open surgical approaches, “closed” endoscopic surgical approaches, pharmacological therapy, and stem cell/gene therapy. Advances in the field have been characterized by alternating exuberance at spectacular successes, but also periods of intense frustration at technical challenges to be overcome to bring new approaches on line. “Moving goal posts” have also been a common problem, secondary to improvements in ancillary care that keep raising the bar to show intervention as having a positive benefit to risk ratio.
Even after four decades since the first transfusions by Lilley, the single most misunderstood and continuous issue about fetal therapy continues to be “why before and not after birth.” There is no one single answer; rather there are multiple disorder-specific considerations. If something cannot be treated safely postnatally, then there is generally justification for prenatal intervention. However, for many conditions profound and irreparable damage occurs before birth, making fetal intervention the best or sometimes only way to ameliorate the damage. Some procedures have been quite rare. Others are more common. The expectation is that with improvements and increasing utilization of prenatal diagnosis, more women will choose to consider the opportunities to treat fetuses before birth.
SURGICAL THERAPY
In Utero “Closed” Fetal Surgery
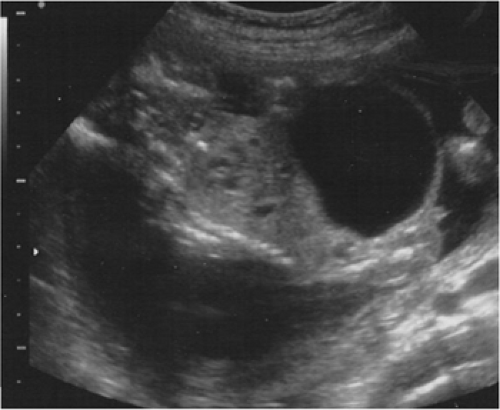
The most successful in utero fetal surgery has been for the evaluation and treatment of obstructive uropathy (5,6). Lower urinary tract obstruction (LUTO) is a heterogeneous entity that affects 1:500—8,000 newborn males (5,6,7,8). Posterior urethral valves or urethral atresias are the most common causes, although stenosis of the urethral meatus, anterior urethral valves, ectopic insertion of a ureter and tumors of the bladder have also been observed. Massive distention of the bladder can be seen with compensatory hypertrophy and hyperplasia of the smooth muscle within the bladder wall. Loss of compliance and elasticity, and poor postnatal function generally require post natal surgical reconstruction (9). Elevated intravesicular pressures prevent urine inflow from the ureters, eventually distortion of the ureterovesical angles contributes to reflux hydro-nephrosis (5,6,7). Progressive pyelectasis and calyectasis compress the delicate renal parenchyma within the encasing serosal capsule, leading to functional abnormalities within the medullary and eventually the cortical regions (5,6,7,8,9,10). Focal compressive hypoxia likely contributes to the progressive fibrosis and perturbations in tubular function resulting in urinary hypertonicity. Obstructive processes can eventually lead to type IV cystic dysplasia and renal insufficiency (8,9).
The effects extend beyond the genitourinary tract. Progressive oligo/anhydramnios leads to compressive deformations as seen in Potter sequence, including extremity contractures, facial dysmorphology, and disruptions of abdominal wall musculature, as in prune belly. Absence of normal amniotic fluid volume profoundly impedes pulmonary growth and development. Constant compressive pressure on the fetal thorax leads to restriction of expansion of the chest through normal physiological “breathing movements.” Babies born with LUTO mostly die because of pulmonary complications. They do not live long enough to die of renal failure.
Sonographic findings in LUTO include dilated and thickened walls of the bladder, hydronephrosis, and oligohydramnios (Fig. 13-1). Urethral strictures or atresia, urethral agenesis, megalourethra, ureteral reflux, and cloacal anomalies may be present and have a very similar appearance on ultrasound. The typical “keyhole sign” of proximal urethral dilation is secondary to urethral obstruction present in posterior urethral valves or atresia. However, the precise diagnosis can only be made after birth (9).
The prenatal evaluation and management of fetuses with the sonographic findings of LUTO require multiple steps (5,6,7,8). Ruling out other congenital anomalies such as cardiac and neural tube defects is necessary before intervention can be considered.
Karyotyping is essential to confirm a normal male chromosomal status. Most are isolated problems, but the incidence of aneuploidy is higher than the general population. Female fetuses, however, almost always have more complex syndromes of cloacal malformations and do not benefit from in utero shunt therapy. Because of the presence of oligo/anhydramnios, we commonly obtain karyotypes by transabdominal chorionic villus sampling, which gives reliable results within several days during which the remainder of the prenatal evaluation is underway. Fluorescence in situ hybridization is now commonly used to get rapid status of chromosomes 13, 18, 21, X & Y (11,12).
Essential to the prenatal workup is the evaluation of underlying renal status in the fetus. Over the past 15 years, a multicomponent approach has developed for the analysis of fetal urine that evaluates proximal tubular and possible glomerular status using sodium, chloride, osmolality, calcium, β-2 microglobulin, albumin, and total protein concentrations (6,8). It has been shown to be significantly improved by sequential samplings at 48- to 72-hour intervals to be useful approach. The degree of impaired renal function and damage with the extent of urinary hypertonicity and proteinuria can then be directly correlated. The ability to counsel patients about the renal status of their fetus and the long-term prognosis has been dramatically improved as a result.
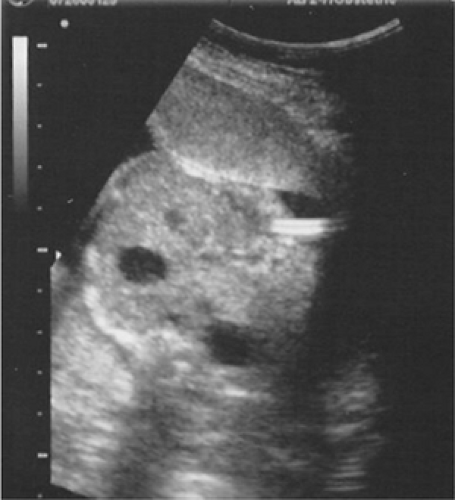
Vesicoamniotic catheter shunts bypass the urethral obstruction diverting the urine into the amniotic space to allow appropriate drainage of the upper urinary tract and prevention of pulmonary hypoplasia and physical deformations (Fig. 13-2). In fetuses with isolated LUTO, a normal male karyotype, and progressively improving urinary profile that meet threshold parameters (Table 13-1), intervention has been very successful in salvaging fetuses using percutaneous vesicoamniotic shunt therapy.
Subsequent experience in humans has been widely variable and appears to be related to the extent of prenatal evaluation prior to shunt placement, and the etiology of obstruction. Freedman and associates found that prune
belly infants, without complete urethral obstructions have very good renal outcomes following vesicoamniotic shunt therapy (9). They also found significant improvement in survival and renal function in infants with posterior urethral valves treated by shunting. However, many such children develop mild-to-moderate renal insufficiency at birth and several of these have progressed to renal failure, requiring dialysis, and transplantation. The worst prognosis appears to be for those with urethral atresia. There have been survivors, including some with urethral atresia, following early shunt intervention. Animal studies have indicated that early onset, complete obstructions result in more severe renal damage than later onset or partial obstructions. Such data emphasizes the necessity of early diagnosis, evaluation, and intervention to achieve the best outcomes in such cases.
belly infants, without complete urethral obstructions have very good renal outcomes following vesicoamniotic shunt therapy (9). They also found significant improvement in survival and renal function in infants with posterior urethral valves treated by shunting. However, many such children develop mild-to-moderate renal insufficiency at birth and several of these have progressed to renal failure, requiring dialysis, and transplantation. The worst prognosis appears to be for those with urethral atresia. There have been survivors, including some with urethral atresia, following early shunt intervention. Animal studies have indicated that early onset, complete obstructions result in more severe renal damage than later onset or partial obstructions. Such data emphasizes the necessity of early diagnosis, evaluation, and intervention to achieve the best outcomes in such cases.
TABLE 13-1 UPPER THRESHOLD VALUES FOR SELECTING FETUSES THAT MIGHT BENEFIT FROM PRENATAL INTERVENTION FOR URINARY OBSTRUCTION | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Data from our experience over the past 15 years suggest that patients having bladder shunts had a 91% survival, but that long-term renal function was not guaranteed. Just under half had “normal renal function,” and about a quarter had mild impairments. The experience depended largely on the exact etiology of the disorder with posterior urethral value having the best outcomes and urethral atresia the worst. Our experience suggests that close pediatric urological/renal function assessment is essential to maximize outcomes. The Paris group has found, consistent with our experience, that about 25% of children had serious, long-term renal impairments and about 15% actually developed end-stage renal disease requiring trans-plant (10).
Although vesicoamniotic shunting has certainly improved survival and renal function in cases of early obstructive uropathy, complications of this procedure remain unacceptably high. We found in the eighties and nineties that in 40% of our cases, the shunts became physically displaced into the amniotic or intraperitoneal space, or have become blocked causing loss of drainage function and necessitating replacement. On balance, intervention for LUTO has clearly saved fetuses, who would otherwise have surely died. Many have normal to moderately impaired renal function. A carefully balanced approach in counseling is required for patients to determine what is right for them.
Other Shunt Procedures
Shunting was attempted for obstructive hydrocephalus, in the late 1970s (13). The results were almost uniformly dismal. Most of the patients operated on were, in retrospect, very poor candidates for intervention. Many had multisystem syndromic disorders, including aneuploidy, and hopeless congenital anomalies such as holoprosencephaly.
Ventriculoamniotic shunts were abandoned the early eighties. However, with a better understanding of the poor natural history of the anomaly, and improved, more accurate diagnostic techniques, there may eventually be some limited applications for prenatal neurological shunting. It may eventually be useful in cases of early onset, isolated, progressive obstructive hydrocephaly.
The other use of percutaneously placed shunts has been for thoracic abnormalities (14,15). The macrocystic form of congenital cystic adenomatoid malformation (CCAM) can present with a very large intrathoracic mass with a dominant macrocyst which causes cardiac and mediastinal shifts. Potential hemodynamic changes can result along with pulmonary compression and risk of lung hypoplasia. Such dominant cysts can be approached using pleuroamniotic shunts to chronically drain these structures, reducing their volume, and diminishing their space occupying effects within the thoracic cavity.
Isolated pleural effusions can enlarge, causing hemodynamic changes and onset of generalized hydrops and pulmonary compression. The mass interferes with normal lung development increasing the risk of hypoplasia. Small, unilateral effusions generally do not warrant intervention, but cannot be ignored as they have the potential for rapid progression (15).
As with all fetal interventions, prenatal evaluation prior to intervention is critical for appropriate case selection. Seemingly isolated effusions may be associated with cardiac malformations, aneuploidy, anemia, or an infectious process. Thoracoamniotic shunting is effective in carefully evaluated cases when the risk of pulmonary hypoplasia from large effusions early in gestation is present, or early signs of progressive hydrops (unilateral or bilateral effusion, skin or scalp edema, ascites, pericardial effusion) appear. Fetal anemia, per se, is not an indication for thoracoamniotic effusion shunting. Hydropic changes will usually resolve with timely fetal transfusion therapy. By the time the fetus develops significant ascites, the prognosis even with successful shunt intervention, diminishes considerably (15). However, several cases with suddenly progressive pleural effusions and onset of generalized hydrops have been treated with complete resolution of all hydropic complications and normal postnatal infants.
Open Surgery
Open fetal surgery has been performed for a limited number of indications for nearly two decades (3). There are appropriate concerns for maternal risks, rigorous selection criteria, and somewhat frustrating results. There has been continuing innovation and development of instruments and techniques, motivated by the clinical necessity to improve the safety of open fetal surgery for both the fetus and the mother, which have turned the field upside down in many instances.
Congenital Diaphragmatic Hernia
The surgical approach to congenital diaphragmatic hernia (CDH) has evolved considerably since the first attempted CDH repair in 1986 and the first success in 1989 (16,17,18). Definitive repair of CDH by reduction of viscera from the chest, diaphragmatic patch placement, and abdominal silo construction (to reduce intraabdominal pressure) satisfied the desire for a single step approach but had unacceptable
mortality, particularly after a clinical trial showed that open fetal repair was no better than postnatal repair when the liver was herniated into the chest (19,20). The definitive repair was then abandoned and in utero tracheal occlusion took its place (Fig. 13-3) (20,21,22,23,24,25,26).
mortality, particularly after a clinical trial showed that open fetal repair was no better than postnatal repair when the liver was herniated into the chest (19,20). The definitive repair was then abandoned and in utero tracheal occlusion took its place (Fig. 13-3) (20,21,22,23,24,25,26).
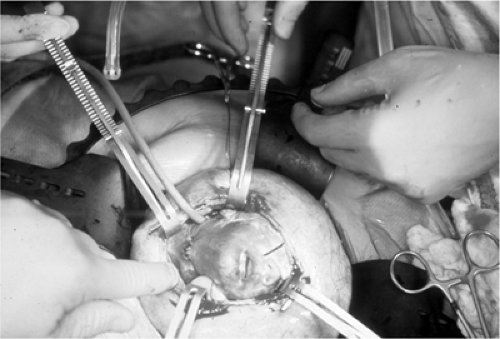 Figure 13-3 Surgical isolation of the fetal trachea prior to placement of hemoclips in a tracheal occlusion procedure for congenital diaphragmatic hernia. (See color plate) |
Tracheal occlusion produces increased lung size through accumulation of pulmonary secretions. The herniated viscera are reduced from the chest and therefore decrease the risk for lung hypoplasia. The technique of achieving reliable, complete and reversible tracheal occlusion has evolved. Initially, it could only be accomplished by open fetal surgery and fetal neck dissection (taking care to avoid the recurrent laryngeal nerves) and placement of occlusive hemoclips. Then, a fetoscopic technique was developed to accomplish the same neck dissection and tracheal clip (the Fetendo Clip Procedure) (24). While successful, it proved difficult, with a significant learning curve. Attempts to simplify the procedure by developing an appropriate polymer to use as a tracheal plug inserted through the fetal mouth have been generally unsuccessful. Unless there was complete occlusion, the pulmonary secretions would leak, thereby defeating the purpose of the plug. Finally, a relatively simple technique was developed in which a fetoscope passed through a single port is advanced into the fetal trachea (fetal bronchoscopy) and a detachable silicone balloon is inflated to occlude the trachea.
CDH has a prototype of rapid changes in technology, further clouding any simple attempts to understand the role for fetal surgery. With increasing sophistication of the surgical approach and concomitant improvements in neonatal care using extracorporeal membrane oxygenation, it was impossible to accurately determine the relative benefits of each approach without a prospective, randomized comparison that held all other details constant. Thus, after much debate, a randomized trial of surgery for CDH vs. optimal postnatal care was funded by National Institute of Child Health and Human Development (NICHD) (21). The principal component of the trial was that patients in the postnatal care arm (control group) would receive the same neonatal care by the same center as the surgical arm.
It was originally expected that patients having the surgery would have a survival rate of about 70%. The best data on controls showed survivals of about 35%. The surgically treated patients achieved the expected survival rate. However, by having the controls cared for at the same tertiary specialty centers as the surgical group, survival in the controls was essentially the same as the surgical group. Therefore, the trial was stopped prematurely. Such data show dramatically the principle of “the moving target” and how our use of technology must continually adapt to changing conditions (27).
Congenital Cystic Adenomatoid Malformation
CCAM is a space occupying congenital cystic lesion of the lung. Hydrops evolves by causing mediastinal shift which compromises venous return to the heart. When fetuses with CCAM develop hydrops, the fetal mortality approaches 100% (Fig. 13-4) (28,29,30). Fetal resection of CCAM can reverse hydrops and has improved survival dramatically (17). The fetal operation is performed by exposure of the arm and chest wall on the side of the lesion through the maternal hysterotomy. A large muscle sparing thoracotomy is performed through the midthorax of the fetus and the lobe containing the CCAM is isolated. The attachments of
the lobe to adjacent lung tissue are bluntly divided and the lobar hilum is divided by use of a stapler or a bulk ligature. During the remainder of the pregnancy, the remaining normal lung shows compensatory growth to fill the space left following removal of the mass.
the lobe to adjacent lung tissue are bluntly divided and the lobar hilum is divided by use of a stapler or a bulk ligature. During the remainder of the pregnancy, the remaining normal lung shows compensatory growth to fill the space left following removal of the mass.
Sacrococcygeal Teratoma
Fetal sacrococcygeal teratoma (SCT) arises from the presacral space, which may grow to massive proportions and in some fetuses induces high output congestive heart failure from tumor vascular steal. Fetal SCT with high output physiology and associated placentomegaly or hydrops uniformly results in fetal demise (Fig. 13-5) (31,32). The pathophysiological rationale for fetal surgery is to ligate the vascular connections to the tumor, remove the vascular shunt, and reverse the high output physiology. The fetal operation is performed by exteriorization of the fetal buttocks with attached tumor (31). The head, torso, and lower extremities of the fetus are kept in utero if at all possible. Since the tumor can sometimes be larger than the fetus, significant loss of uterine volume occurs, and the uterus may contract increasing the risk for placental abruption, placental dysfunction because of compression, or postoperative preterm labor. Once exteriorized, the anus is identified, and the fetal skin is incised posterior to the anorectal sphincter complex to avoid injury to the continence mechanism. A tourniquet is then applied at the base of the tumor and brought down gradually as the tumor is finger fractured down to its vascular pedicle. The vascular pedicle is then ligated or stapled depending on the width of the pedicle. The entire fetal procedure can be performed in less than 15 minutes with minimal blood loss. Because of the increase in afterload following ligation of the low resistance tumor circuit, the fetal hemodynamic status must be monitored by fetal echocardiography during and in the immediate period following the ligation.
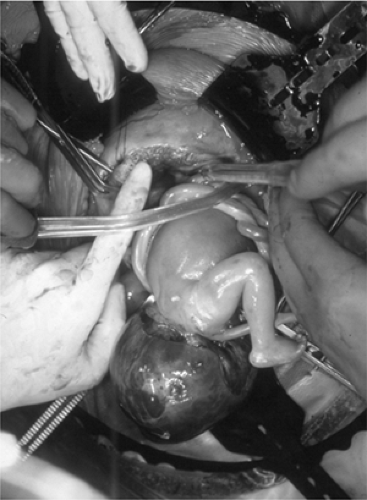 Figure 13-5 Saccrococcygeal teratoma in a fetus. (See color plate) |
Neural Tube Defects
Neural tube defects (NTDs) result from abnormal closure of the neural tube which normally occurs between the third and fourth week of gestational age. The etiology is complex, with both genetic and environmental factors involved. There are historical data in humans suggesting increased NTD frequencies in subjects with poor dietary histories or with intestinal bypasses. Analysis of recurrence patterns within families and of twin-twin concordance data provides evidence of a genetic influence in nonsyndromal cases. However, factors such as socioeconomic status, geographic area, occupational exposure, and maternal use of antiepileptic drugs are also associated with variations in the incidence of NTDs (33). In 1980, Smithells and associates suggested that vitamin supplementation containing 0.36 mg folate could reduce the frequency of NTD recurrence by sevenfold (33,34,35). For almost a decade, there has been a great deal of controversy regarding the benefit of folate supplementation for the prevention of NTDs3 (36,37,38,39). In 1991, a randomized double-blinded trial designed by the Medical Research Council Vitamin Study Research Group demonstrated that preconceptual folate reduces the risk of recurrence in high risk patients (40). Subsequently, it was shown that preparations containing folate and other vitamins also reduce the occurrence of first time NTDs (41). In response to these findings,
guidelines were issued calling for consumption of 4.0 mg/day folic acid by women with a prior child affected with an NTD, for at least 1 month prior to conception through the first 3 months of pregnancy. Additionally, 0.4 mg/day folic acid is recommended to all women planning a pregnancy to be taken preconceptually. The data on NTD recurrence prevention is now very well established, and has become routine practice for high-risk cases. As of January 1998, the United States Food and Drug Administration have mandated that breads and grains be supplemented with folic acid. The impact of food fortification with folic acid on NTDs birth prevalence during the years 1990-1999 was evaluated by assessing birth certificate reports before and after mandatory fortification (42). It was found that the birth prevalence of NTDs reported decreased by 19%. It is important to note that the continuing decline in NTDs rates are estimated to be as a result of the introduction and increased utilization of prenatal diagnosis in addition to the recommendation for multivitamin use in women of childbearing age and the population-wide increases in blood folate levels because food fortification was mandated (43). Recently, Evans and associates have shown a 32% drop in high maternal serum alpha-fetoprotein (MSAFP) values in the United States comparing 2000 values versus 1997 before the introduction of folic acid supplementation (44).
guidelines were issued calling for consumption of 4.0 mg/day folic acid by women with a prior child affected with an NTD, for at least 1 month prior to conception through the first 3 months of pregnancy. Additionally, 0.4 mg/day folic acid is recommended to all women planning a pregnancy to be taken preconceptually. The data on NTD recurrence prevention is now very well established, and has become routine practice for high-risk cases. As of January 1998, the United States Food and Drug Administration have mandated that breads and grains be supplemented with folic acid. The impact of food fortification with folic acid on NTDs birth prevalence during the years 1990-1999 was evaluated by assessing birth certificate reports before and after mandatory fortification (42). It was found that the birth prevalence of NTDs reported decreased by 19%. It is important to note that the continuing decline in NTDs rates are estimated to be as a result of the introduction and increased utilization of prenatal diagnosis in addition to the recommendation for multivitamin use in women of childbearing age and the population-wide increases in blood folate levels because food fortification was mandated (43). Recently, Evans and associates have shown a 32% drop in high maternal serum alpha-fetoprotein (MSAFP) values in the United States comparing 2000 values versus 1997 before the introduction of folic acid supplementation (44).
Folate plays a central part in embryonic and fetal development because of its role in nucleic acid synthesis mandatory for the widespread cell division that takes place during embryogenesis. Folate deficiency can occur because of low dietary folate intake or because of increased metabolic requirement as seen in particular genetic alterations such as the polymorphism of the thermolabile enzyme methyltetrahydrofolate reductase (MTHRF). However, evidence regarding its role in NTD is unsupported, except in certain populations, suggesting that these variants are not large contributors to the etiology of NTDs (45,46). Additional candidate genes other than MTHFR may be responsible for an increased risk for NTDs (47). It has recently been reported that methionine synthase polymorphisms are associated with increased risk for NTDs, that is not influenced by maternal preconception folic acid intake at doses of 0.4 mg/day (48). Other candidate genes include the mitochondrial membrane transporter gene UCP2 (49). Despite previous studies suggesting zinc deficiency to play a role in the etiology of NTDs (50,51), further studies were inconclusive (52,53). Because methionine deficiency may be involved in NTDs, it may be beneficial in NTD risk reduction (54). Preconception folic acid intake as a sole vitamin or as multivitamin supplementation reduces the risk of recurrence and first time NTDs.
Babies with meningomyeloceles have impaired lower motor function, loss of bowel, and bladder control. A significant percentage develop obstructive hydrocephalus, which requires ventriculoperitoneal shunting (55,56). Experience from the 1970s and 1980s showed that babies with meningomyelocele delivered atraumatically by cesarean section had a better level of motor function for the given level of anatomic defect, than those babies delivered through the vaginal canal (55). Such data suggest that compression and trauma to the cord in the delivery process can have permanent long-term sequelae to motor function. In theory, trauma to the spinal cord in utero, either from banging into the uterine wall or the toxic effects of the amniotic fluid in the third trimester, could be detrimental to the function of the spinal cord. Traditional dogma held that the pathogenesis of meningomyelocele was that an abnormally developed spinal cord, which did not engender the proper development of the bony spinal column, may not be the whole story. It is possible that the primary defect is in the bony spinal column, which exposes a presumptively undamaged spinal cord. The cord is then damaged by the toxic affects of amniotic fluid and trauma from the uterine environment and repeated contact with the uterine wall. Thus, the rationale for attempts to cover and protect the spinal cord in utero, to minimize the seque-lae (57).
Three groups (58,59,60) have done most of the work in this area and have attempted to repair meningomyeloceles in utero, both as an open surgical procedure, and endoscopically, with the stated attempt to reduce long-term morbidity and mortality. The principal benefit of the surgery is likely secondary, i.e., a significant reduction in the number of babies requiring ventriculoperitoneal shunting for obstructive hydrocephalus (58,61). There is still much controversy surrounding the data (54). A randomized, prospective trial comparing fetal to postnatal neurosurgical closure began in 2003 but will take several years to be completed. A major milestone of this trial has been the agreement among the participating centers not to perform any cases outside the trial, and other centers around the country have agreed not to start programs until the trial is completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree