Halsby et al., 2014[6]
RCOG Green-Top Guideline No. 13, 2007[9]
(antibodies not protective)
Clinical presentations
There are essentially two ways in which fetal infection can present as a possibility to fetal medicine specialists.
1. Identification of primary maternal infection in pregnancy or a positive screening test
Screening for rubella and syphilis antibodies is part of booking investigations. The presence of rubella antibodies is evidence of maternal immunity and that the mother is protected. The absence of rubella antibodies implies that the mother is susceptible to the infection. Immunization is recommended after delivery. Syphilis screening tests need to be followed by further diagnostic tests to confirm infection and assess its stage as well as any potential infectivity and risk to the unborn child. Maternal treatment of syphilis is effective in preventing congenital syphilis and its fetal sequels.
Sometimes, maternal infection can be diagnosed following signs and symptoms. Varicella and parvovirus infection in the mother are commonly diagnosed due to the presence of a skin rash. Varicella skin lesions are characteristic. Both varicella and parvovirus infections can be confirmed by maternal serology showing a positive IgM, and negative IgG specific antibodies. Both these infections are generally self-limiting in the mother. Rarely, maternal varicella can be complicated by varicella pneumonia or encephalitis, which are serious conditions. Maternal CMV or toxoplasma infections are uncommonly diagnosed because of nonspecific signs and symptoms. Primary maternal infection is confirmed by evidence of maternal seroconversion, and the presence of positive IgM and negative IgG specific antibodies in maternal serum. Testing of booking blood showing negative results for both IgG and IgM antibodies with later positivity for these antibodies is a reliable evidence of seroconversion.
Performing a TORCH (toxoplasma, rubella, CMV and herpes) test without a clear indication is generally not recommended. The test detects antibodies but is unable to distinguish between a recent and a past infection. The majority of positive test results are as a result of past infection.
2. Ultrasound features that raise the possibility of fetal infection as the underlying cause
Some ultrasound features raise the suspicion of fetal infection as the underlying cause[15]. Table 14.2 shows suspicious ultrasound features and possible responsible agents.
| Ultrasound feature | Possible infection |
|---|---|
| Hydrops fetalis | Parvovirus, rubella, varicella, CMV, toxoplasmosis |
| Ventriculomegaly | CMV, toxoplasmosis |
| Microcephaly | CMV, toxoplasmosis, syphilis, rubella |
| Intrauterine growth restriction | CMV, rubella, syphilis, toxoplasmosis |
| Placentomegaly | Parvovirus, syphilis, toxoplasmosis |
| Cardiomegaly and hyperdynamic circulation | Parvovirus |
| Calcifications | CMV, syphilis, toxoplasmosis |
| Limb deformities | Varicella, syphilis |
CMV: cytomegalovirus.
Identification of a positive TORCH test
A common practice is to perform a TORCH test, this being testing without a clear reason. These tests are not carried out as part of routine antenatal investigations. Serology for rubella and syphilis is tested at booking.
Serologic investigations
In general, the first step in cases of suspected fetal infection is to explore if the mother shows evidence of recent infection (Table 14.3). Fetal infection is not possible without maternal infection, but all maternal infections are not passed on to the fetus. The first response after contacting an infection is the production of IgM antibodies. This is followed by production of IgG antibodies. Both these antibody types are specific to the infecting organism. Newly formed IgG antibodies are less ‘sticky’ to the antigen, and show a low (<30%) avidity. Low-avidity antibodies are suggestive of a recent seroconversion. Over time, the IgG antibodies mature and the avidity is high (>70%). High-avidity antibodies are suggestive of seroconversion >6 months prior.
| IgG | IgM | Interpretation | Action |
|---|---|---|---|
| Negative | Negative | Mother susceptible but not infected | Reassure Suggest precautions to prevent infection |
| Negative | Positive | Possible acute infection (primary) | Test booking blood Repeat serology in a few days |
| Positive | Negative | Mother immune, infection unlikely | Reassurea |
| Positive | Positive | Likely primary infection | Test booking blood Perform IgG avidity |
a Test booking blood. Perform IgG avidity if the clinical suspicion is strong or test is performed several weeks after booking.
If the mother showed primary infection in the pregnancy, fetal infection is a possibility. At this stage, the probability of fetal infection and its implications should be explained to the parents. Fetal infection can be tested by amniocentesis/fetal blood sampling after some time (making allowance for the time it takes to reach the fetus) if the parents request it. Fetal infection can be detected by looking for the specific antigen of the infecting agent. Implications of fetal infection can be a wide spectrum from being asymptomatic to severe damage.
Cytomegalovirus
CMV is the most common cause of fetal infection occurring in 3–4 per 1,000 live births with significant racial variation. Pregnant women usually acquire CMV infection by exposure to children in their home or from occupational exposure to children. Infection can potentially occur from close contact with body fluids, such as saliva, blood, semen, urine and cervical secretion. Maternal infection is usually asymptomatic and the mother is generally unaware of being infected with CMV. A small proportion of patients may experience symptoms such as malaise, fever, generalized lymphadenopathy and hepatosplenomegaly.
Routine screening for maternal CMV seroconversion during pregnancy is not recommended in the UK. In some countries, such as Italy and France, serologic screening is performed in the first half of pregnancy. The main argument for not offering routine screening is lack of an effective treatment for reducing the rate of fetal transmission.
Fetal infection with CMV is a result of a transplacental passage of the virus during primary maternal CMV infection. Reinfection is uncommon and can be the result of a reactivation of the virus in the host or reinfection with a different CMV strain. The outcome of the fetus/newborn is directly correlated to the type and timing of infection. The most severe forms occur as the result of a primary infection contracted in the first 2 months of pregnancy.
The risk of transmission from mother to fetus is 30–50% during a primary infection. Of those infected, 90% of the fetuses are usually asymptomatic at birth, while 10% may manifest signs or symptoms related to CMV infection. Of the 10% of fetuses showing signs of infection, a third experience a fetal or neonatal demise. Among the survivors, half present major sequelae. Among asymptomatic fetuses, 10% may experience sensorineural hearing loss. Transmission from mother to fetus is considerably lower in a nonprimary infection and occurs in around 1% of the cases. The course of fetal disease is no different in primary or secondary maternal infection once infection has established.
The following diagram shows a mathematical modeling of a hypothetical cohort of 1,000 pregnant women with primary CMV infection (Figure 14.1).
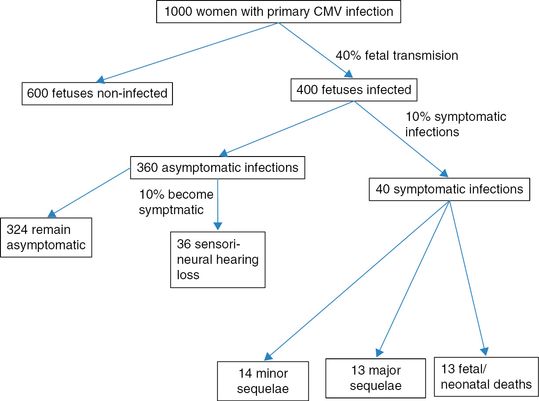
Laboratory investigations. CMV: cytomegalovirus.
The gold standard of serologic diagnosis of CMV infection is maternal seroconversion or the presence of anti-CMV IgM antibodies combined with anti-CMV IgG antibodies of low avidity. When a serum sample from early pregnancy is available, the diagnosis of CMV is facilitated by the documentation of seroconversion to CMV-specific antibodies in a previously seronegative woman.
A rise in IgM antibody titer may indicate a reactivation or reinfection with CMV. The rate of vertical transmission during a nonprimary infection is significantly lower compared with a primary infection. Moreover, IgM antibodies can be produced for months after the acute phase of infection. An increase in IgG antibody titre with or without the presence of IgM antibodies and high avidity is suggestive of a nonprimary CMV infection.
Ultrasound features of CMV infection
CNS (ventriculomegaly, microcephaly, intraventricular hemorrhage, intraventricular adhesions, periventricular echogenicities, lissencephaly, porecephaly, cerebellar agenesis/hypoplasia, microphthalmia)
Intrauterine growth restriction (IUGR)
Placental enlargement
Gastrointestinal tract (hepatomegaly, splenomegaly, echogenic bowel, intra-abdominal and liver calcification, ascites)
Heart (cardiomegaly, pericardial effusions and calcifications)
Hydrops fetalis
A serologic assessment of the mother is warranted when dealing with a fetus presenting with apparently isolated ventriculomegaly at the scan, particularly if a recent history of flu-like symptoms is reported. Periventricular intracranial calcifications are also relatively commonly associated with congenital CMV infection. Although in the presence of CMV infection fetal brain malformations are common, isolated extracerebral abnormalities can be detected in around 30% of the cases. Echogenic bowel and bowel dilation are the most common extracerebral findings detected at the scan in an infected fetus and are the result of viral enterocolitis. Hepatosplenomegaly and multiple liver calcifications can also be seen.
Although ultrasound has a low sensitivity in detecting congenital CMV infection even in patients at risk, the presence of ultrasound signs is an independent, poor prognostic factor. Therefore, a detailed ultrasonographic assessment in centers of excellence is warranted. It is important to state that a negative ultrasound scan in the second trimester does not exclude fetal infection. Magnetic resonance imaging (MRI) has a better spatial resolution compared with ultrasound, and may be helpful in detecting additional brain abnormalities, especially late in gestation.
Congenital CMV infection may cause a wide range of neurologic disabilities, including mental retardation, cerebral palsy, autism, epilepsy, blindness or visual deficits and learning disabilities. CMV is also the main cause of sensorineural hearing loss during childhood, which is present in about 10–15% of all infected babies, with the risk being greater if the infection was symptomatic in the neonatal period. See also Table 14.2.
Management
Confirmation of fetal infection through an invasive test (amniocentesis or fetal blood sampling) should be offered to the parents. Fetal infection is very unlikely if CMV is not detected in a fetal sample obtained at least 6–8 weeks after maternal infection or after 20 weeks of gestation. Isolation of CMV in the amniotic fluid by polymerase chain reaction (PCR) confirms the diagnosis of fetal infection, but does not predict the outcome of these babies at birth. In this case, viral load assessment through the use of quantitative PCR may be useful. A high viral load in the amniotic fluid (defined as the presence of >103 copies/mL) is likely to indicate a symptomatic infection. Recently, several authors have tried to correlate antenatal virologic, biochemical, hematologic and imaging parameters with the likelihood of an adverse outcome following confirmed fetal infection using fetal blood biochemistry. Fetal thrombocytopenia, elevated liver enzymes and abnormal findings at ultrasound correlate with poor neonatal outcome.
There is currently no proven prenatal treatment for congenital CMV infection. The use of CMV hyperimmune globulin or antiviral drugs given to the mother has been proposed to reduce the course of infection and the rate of vertical transmission. In a non-randomized prospective study, it was reported that maternal treatment with hyperimmune globulin was associated with a lower chance (3%) of symptomatic babies compared to 50% of the control group[11]. In another study it was proposed that antenatal valaciclovir given to the mother reduces the viral load in fetal blood. Randomized clinical trials are needed in order to clarify the role of antenatal treatment with immunoglobulins or antiviral drugs in congenital CMV infection. Postnatal diagnosis of congenital CMV infection relies on the isolation of the virus in the urine or saliva of the baby in the first 2 weeks of life. Symptoms of congenital CMV infection include hepatosplenomegaly, jaundice and petechiae. Neurologic symptoms include hypotonia, lethargy and seizure. Not all newborns presenting with symptoms related to congenital infection have a poor neurologic outcome and one-third of these babies have been reported not to have severe neurologic impairment. Microcephaly and abnormal brain findings at postnatal imaging are highly correlated with adverse neurodevelopmental outcome. Babies with a confirmed congenital CMV infection should be assessed sequentially during childhood by a multidisciplinary team including a pediatric neurologist, ophthalmologist and ENT surgeon. Figures 14.2 and 14.3 show the above discussion diagrammatically.
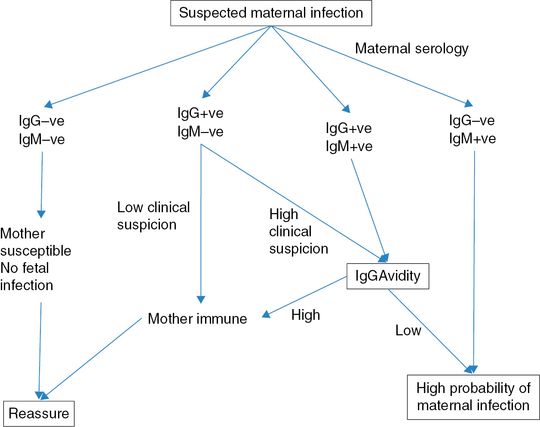
Management of women with suspected cytomegalovirus infection. IgG: immunoglobulin G IgM: immunoglobulin M..
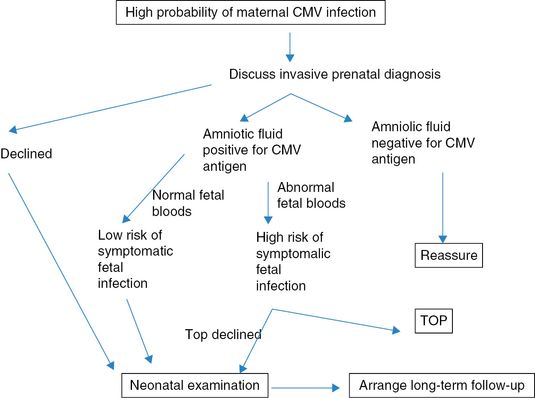
Management of women with serology suggesting a high probability of cytomegalovirus (CMV) infection. TOP: termination of pregnancy.
Key counseling points
Maternal infection with CMV is often asymptomatic.
The greatest risk to the fetus is with a primary CMV infection in early pregnancy (first trimester).
The risk of fetal infection following maternal infection is 30–40%.
Fetal infection can be tested by an invasive test (amniocentesis/fetal blood sampling). Amniotic fluid sample is more reliable for the detection of CMV antigen.
A majority (90%) of congenitally infected fetuses are asymptomatic at birth.
There are no reliable tests to exclude symptomatic fetal infection. However, abnormal ultrasound features (particularly microcephaly, ventriculomegaly) or abnormal fetal brain MRI are highly suggestive of developmental delay.
Abnormal fetal liver function tests or presence of anemia or thrombocytopenia in the fetal blood are also suggestive of a guarded prognosis.
No drug has a proven effectiveness to prevent or limit fetal damage.
Toxoplasmosis
Toxoplasma gondii is an obligate intracellular protozoan, whose lifecycle is characterized by a sexual phase occurring only in cats and an asexual phase taking place within virtually all warm-blooded animals, including humans. The mother acquires infection by ingestion of uncooked or raw meat containing Toxoplasma cysts. Water or food contaminated by oocysts excreted in the feces of infected cats represents another source of infection. After ingestion, Toxoplasma acquires its active form and invades intestinal epithelial cells, thus spreading in the circulation.
Maternal infection is usually asymptomatic and only a small proportion of women develop low-grade fever, malaise or lymphadenopathy. Rarely, Toxoplasma infection manifests primarily as chorio-retinitis. Transmission from the mother to fetus occurs almost exclusively during a primary maternal infection. The frequency of vertical transmission increases with the gestational age and is around 70% in the third trimester, while being as low as 5–6% in the first trimester of pregnancy. Risk of fetal infection with toxoplasmosis and the risk of being symptomatic if infected is shown in Figure 14.4 [13]. At no point is the overall risk of a symptomatic infection less than 5–8%.
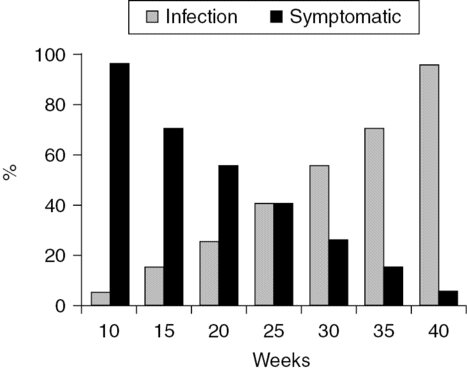
Risk of vertical transmission (gray bars) with toxoplasmosis and the risk of being symptomatic if infected (dark bars).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


