Baseline Rate
The normal fetus exhibits an overall constancy in its heart rate that can be considered comparable in nature to the resting heart rate of an adult. Most people maintain a certain average heart rate at rest. Although that rate changes with activity or excitement, it is expected to return to its usual rate when such stimuli are not present. This is also true of the fetus.
As explained in detail in
Chapter 1, the FHR is controlled by a number of mechanisms. These include the central nervous system (CNS), the autonomic nervous system (ANS), baroreceptors, chemoreceptors, and the endocrine system. As was also explained previously (see
Chapter 2), the recorded trend of the FHR is calculated from the time intervals that elapse between successive beats of the fetal heart. Normally, this interval varies slightly from one beat to the next, causing a certain degree of fluctuation to appear in the recorded trend of the baseline FHR. The bulk of these fluctuations are normally consolidated within a relatively constant and discernible plotting of data that is recognized as the
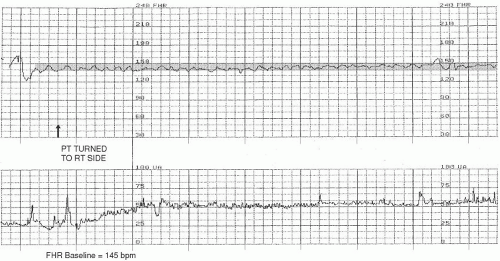
FHR baseline. Normal FHR baseline falls within the range of 110 to 160 beats per minute (
Figure 3-1). According to the NICHD
4 definition, FHR baseline is “determined by approximating the mean FHR rounded to increments of 5 beats per minute (bpm) during a 10-minute window, excluding accelerations and decelerations and periods of marked FHR variability (>25 bpm).” Often, this integer is quickly determined by visual assessment. If the FHR baseline is plotted over a visually wider range, it may be helpful to actually calculate the mean and then round off; for example, if the bulk of the FHR baseline data is visualized as plotted on the fetal strip chart between 125 to 147 bpm, the mean FHR would be arrived at by adding these two integers (125 + 147) and dividing the result by 2 (272 ÷ 2). The mean (in this case, 136) would then be rounded to the nearest increment of 5 (in this case, 135), if necessary. This method of rounding-off is not meant to prompt an intricate process of mathematical calculation; to the contrary, it was intended to encourage simple and practical visual assessment and interpretation.
To discern a trend in the FHR that can be interpreted as the baseline, it is necessary to observe the recording of the FHR over a period of time. The FHR is usually assessed over a minimum of 10 minutes.
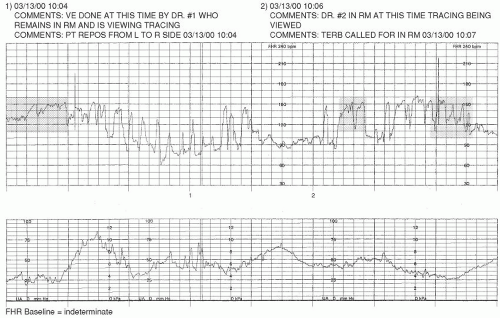
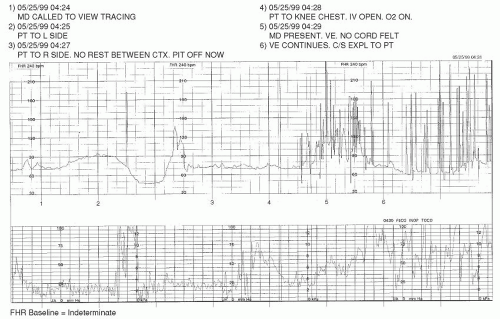
It is important to know that there may be periods during which the FHR baseline cannot be assessed and/or interpreted. As the NICHD
4 definition of FHR baseline states, “excluding accelerations and decelerations and periods of marked FHR variability (>25 bpm).” Accelerations and decelerations are waveform-shaped, transient excursions of the FHR that rise above (accelerations) or fall below (decelerations) the determined FHR baseline and are visually apparent on analysis of the FHR tracing. These waveforms may occur directly in response to contractions (referred to as a
periodic changes), and some may result from interventions performed by the care team, fetal movement, or maternal positioning, or arise spontaneously (referred to as an
episodic changes). Accelerations, decelerations, and variability are addressed in detail later in this chapter.
Over the course of any 10-minute portion of the FHR tracing, there must be at least 2 minutes of identifiable baseline data; otherwise, the baseline for that period would be considered
indeterminate. These 2 minutes of data do not need to occur contiguously to be valid for consideration. If the 10-minute snapshot of FHR data being considered does not contain 2 appreciable minutes of FHR baseline data, the clinician is to refer to the previous 10-minute segment(s) to determine the baseline.
4The baseline is the first characteristic of the FHR to be evaluated because it is the parameter against which all other facets of EFM interpretation are based. As mentioned earlier, the baseline is assessed when the FHR is not exhibiting signs of periodic/episodic changes or marked variability. Thus, from a practical standpoint, an optimal time to look for FHR baseline in the laboring patient is in the period immediately prior to the onset of a contraction. Assuming a normal uterine activity pattern, it would be expected that this would be the most likely time when any periodic changes in the FHR, particularly decelerations, would have resolved and the baseline is apt to be most clearly discernible.
Finding the FHR baseline is more than just looking at a segment of the tracing and noting the lowest and highest points where the FHR was recorded. It requires critically evaluating the trend of the FHR at appropriate times to determine the integers between which the bulk of the relevant data are contained. Once the baseline FHR is initially identified for the particular portion of the tracing that is being evaluated, it is useful to reconfirm that finding at other points within the relevant segment of the tracing to ensure accuracy. It is also a good practice to regularly compare observations of the FHR tracing to previously collected data. This process may highlight parameters that may have changed in the interim and help to identify developing trends that may be of concern.
Variability
FHR data is collected in a systematic manner. The first parameter to be assessed is the FHR baseline; the next is FHR baseline variability. As mentioned in
Chapters 1 and
2, there are normal physiologic variations in the time intervals that elapse between each successive beat of the fetal heart. When the FHR is displayed on the strip chart, these variations are visually apparent in the recorded trend of the FHR (see Appendix C). Fluctuation of the FHR baseline is referred to as
variability. Variability is an inherent component of the FHR baseline data and, therefore, can only be assessed during the same periods when it is appropriate to assess the baseline FHR.
Moderate Variability
A moderate amount of FHR baseline variability is considered to be fluctuation in the FHR baseline of as little as 6 bpm to as much as 25 bpm (
Figure 3-2).
1,
2 The presence of
moderate variability indicates that the autonomic and central nervous systems of the fetus are well developed and well oxygenated. Moderate variability is also a reassuring indication that the fetus is maintaining a measure of tolerance to the changes in blood flow that occur during labor. For the period during which it is observed, moderate variability is highly correlated with the absence of significant metabolic acidemia.
2,
5 Attention to and accurate assessment of variability are essential components in the practice of EFM because moderate variability is one of the most important and predictive aspects of the FHR tracing.
Minimal Variability
It may be cause for concern if the FHR baseline appears to have a
minimal variability (≤5 bpm) or
absent variability (amplitude range undetectable)
4 because this finding may signify the presence of fetal hypoxia/acidosis. With minimal or absent variability, there is little or no (respectively) discernible fluctuation in the FHR baseline. It is important to consider this finding within the context of the entire clinical picture to more accurately infer its significance in a particular clinical situation.
As discussed in
Chapter 1, any condition or event that diminishes blood flow to the placenta may deprive the fetus of adequate oxygenation and, if the resulting hypoxemia is sufficient to cause tissue hypoxia and metabolic acidosis, can create a loss of FHR baseline variability. Hypoxic causes of minimal or absent variability are related to diminished blood flow across the placenta or through the umbilical cord. Initially, when such an insult occurs, moderate variability may remain present, indicating that the fetus is effectively compensating for the diminished influx of oxygenated blood. If the cause of decreased perfusion is not corrected; however, variability eventually decreases. This is a warning sign, indicating that the fetus is losing its ability to tolerate or compensate for the stressors placed on it and is becoming hypoxic (
Figure 3-3).
The overall correlation between fetal acidemia and minimal or undetectable FHR baseline variability in the presence of decelerations is only 23%. However, deepening of decelerations (periodic/episodic changes in the FHR, discussed later in this chapter) over time, in association with minimal or undetectable FHR baseline variability, may serve as an indicator for intervention.
2,
5In addition to hypoxia/acidosis, there are other more common reasons for the fetus to exhibit minimal baseline variability. These include fetal sleep, the effects of medications, an immature CNS, fetal dysrhythmias, and cardiac or CNS anomalies. Although fetal sleep is a common cause of minimal variability, it is necessary to remember that this is a transient state that should alternate with periods of moderate variability approximately every 20 to 40 minutes and represents normal fetal physiologic function.
6Medications that depress the maternal CNS are likely to produce similar effects on the fetal CNS. Although it is to be expected that variability will decrease after the administration of such medications, this effect on the fetus is temporary. Once the medication is metabolized and excreted, under normal circumstances, variability will return. It is therefore prudent to administer such medications only after fetal well-being has been established. Also, when decreased variability is noted, it is important to review the patient’s medication history with her because she may have knowingly or unknowingly exposed the fetus to a sedative or narcotic agent. Loss of variability secondary solely to medication effect does not require intervention.
The fetus of less than 32 weeks’ gestation may exhibit less variability because the ANS may not yet be fully developed (see
Chapter 1). It is important to note that, regardless of gestational age, once a fetus has exhibited a certain amount of variability, it has set a standard to which it should be held from that point forward.
Minimal or absent variability is often noted in association with patterns of fetal dysrhythmia. It is also found in the FHR tracings of fetuses with anomalies that affect cardiac, CNS, or ANS functioning. If minimal or absent variability is persistent from the commencement of monitoring and its cause is not known, further investigation is needed (
Figure 3-4). If cardiac or CNS anomalies have not previously been ruled out by sonographic examination, it is advisable to have such testing performed.
Steps should be taken to remediate condition(s) suspected of causing minimal or absent variability. These include increasing fetal oxygenation by optimizing blood flow to and through the uterus, placenta, and umbilical cord. This is accomplished by promoting maternal cardiac and hemodynamic functioning (assess maternal vital signs and history; initiate therapies such as position changes and administration of medications, hydration, and oxygen as needed), uteroplacental perfusion (through maternal positioning and eliminating stress of uterine contractions), and blood flow through the umbilical cord (alleviate pressure through maternal positioning). Research utilizing fetal arterial oxygen saturation monitoring (a method of measuring fetal oxygenation directly) scientifically supported that simultaneous use of IV fluid bolus of 1,000 mL, maternal lateral position, and oxygen administration of 10 L/min with a nonrebreather face mask for 15 minutes improves fetal oxygenation.
7 Not all instances of potential hypoxia can be alleviated with such interventions, but it is optimal to perform them in an attempt to support oxygenation of the fetus. If minimal or absent variability is noted at the outset of the monitoring session and there are no other components of the FHR that can be considered reassuring (such as accelerations, explained later in this chapter), then hypoxia should be promptly ruled out. It is possible that a prior hypoxic insult has occurred (
Table 3-1).
8,
9
Marked Variability
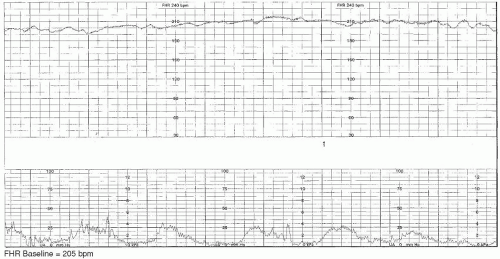
The presence of more than 25 beats of fluctuation in the FHR baseline is known as
marked variability (
Figure 3-5).
4 This pattern is only usually seen intrapartum. Although marked variability is sometimes caused by fetal activity or stimulation, it can also be a sign that the fetus is hemodynamically compromised or mildly hypoxemic.
10 Once again, to put any finding in perspective, it is necessary to consider the entire clinical picture. Query the patient on her perception of fetal activity and attempt to objectively affirm and identify the same. Ensure optimal blood flow to and through the uterus, placenta, and umbilical cord by promoting maternal cardiac and hemodynamic functioning (assess maternal vital signs and history; initiate therapies such as position changes and administration of medications, hydration, and oxygen, as needed). Promote uteroplacental perfusion through maternal positioning and eliminating stress of uterine contractions, and blood flow through the umbilical cord (alleviate pressure through maternal position changes) (
Table 3-2).
Tachycardia
If the baseline FHR is maintained above 160 bpm
4 for 10 minutes or longer, this is considered to be a fetal
tachycardia. A fetal tachycardia may be maternal or fetal in origin. Common conditions that increase maternal heart rate (subsequently raising the FHR) include fever, dehydration, and medication effect. Remediation of the cause of the maternal tachycardia will usually assist the FHR in returning to normal range if the tachycardia is solely of maternal etiology.
Tachycardia may also result from a fetal compensatory response to hypoxia/acidosis, infection, and tachydysrhythmias. The onset of fetal tachycardia or a rising FHR baseline should be considered serious because it is one of the first signs that the fetus is becoming hypoxic/acidotic. As described previously (see
Chapter 1), when there is decreased maternal-fetal perfusion, baroreceptors and chemoreceptors in the
fetus trigger an increase in the FHR. Clinical opportunities to maximize maternal-fetal exchange include avoiding and remediating problems such as tachysystole (
Figure 3-6) and maternal hypotension. In either of these cases, correcting the cause (e.g., discontinuing oxytocin, removal/reversal of uterotonic agents, changing the maternal position) may improve fetal oxygenation and assist the FHR in recovery to its normal range. In the event of infection or tachydysrhythmia, the usual means of intrauterine resuscitation should be employed (i.e., position changes, increased maternal hydration and oxygenation, and decreasing stressors
on the fetus such as contractions) in addition to any other medical or pharmaceutical remedies ordered (
Table 3-3). Although the normal processes of physiologic development (discussed in
Chapter 1) influence the FHR baseline and may cause it to be toward the higher ranges of normal in the preterm fetus, it is important to know that the 110 to 160 bpm baseline FHR range also applies to the preterm fetus. Tachycardia of the FHR baseline in a preterm fetus should be investigated.
Bradycardia
An FHR baseline that is below 110 bpm
4 for a period of 10 minutes or longer is termed a
bradycardia. This can occur in response to a variety of acute and chronic conditions that may be of hypoxic or nonhypoxic etiology. Also, just as it is understood that adults have varying degrees of normal, the same concept applies to the fetus. On occasion, an FHR baseline may be less than 110 bpm but also have reassuring signs present that demonstrate that the fetus is not acidemic (such as moderate variability and/or accelerations), and that rate may be accepted as normal for a particular fetus.
Hypoxic Etiology
The fetus suffering from untreated chronic deprivation of oxygen has a distinct, suspicious-looking tracing.
11 Typically, either variable or late decelerations are present, denoting a decrease of blood flow through either the umbilical cord or the placenta. As the fetus becomes increasingly hypoxic, carbon dioxide accumulates in the fetal blood. This stimulates the chemoreceptors and causes a compensatory elevation in the FHR. As the increase in the FHR baseline continues, FHR baseline variability decreases and any periodic/episodic changes of the FHR that are present may become less visually apparent. The final phase in this process is bradycardia, a terminal event in the case of prolonged fetal hypoxia or asphyxia.
This chain of events is rarely seen in its entirety because surgical intervention usually preempts its completion. It is also possible for the early phases of progressive fetal compromise to be concealed from the clinician because it may have occurred before admission. This clouds assessment of the FHR tracing because there is no normal pattern to which comparison can be made and deviations identified. For instance, the patient may present for fetal monitoring with an FHR that is technically within normal limits and is without decelerations but lacks both accelerations and variability. Interpretation of such a tracing may be confounding and, therefore, it is imperative to promptly determine the status of fetal oxygenation. A biophysical profile may be useful for this purpose. Scalp stimulation, discussed in
Chapter 1, can be
very useful in such an instance, as long as a deceleration or bradycardia is not in progress at the time this method is employed. Fetal scalp stimulation is a simple procedure and may be helpful in determining whether the fetus is presenting to the clinical setting having already sustained neurologic impairment.
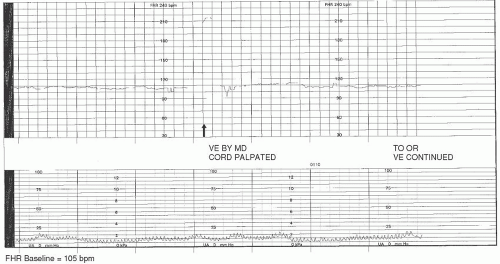
Examples of hypoxic events related to bradycardia include cord prolapse/compression, maternal hypotension, tachysystole, abruptio placentae, or uterine rupture. If the insult that initiated the bradycardia is acute and remediable in nature (such as cord compression, maternal hypotension, and tachysystole) and is identified and treated expeditiously, it is possible that the FHR will return to its normal range. If it does not, a promptly executed cesarean or assisted birth may be indicated.
Cord compression/occlusion can be precipitated by the actions or positioning of the fetus or by such conditions as nuchal cord, cord entanglement, short or knotted cord, occult or overt prolapse, or oligohydramnios. Transient compression of the cord due to fetal position or movement is usually corrected by altering maternal-fetal position. Cord entanglements are not as easily reversible, and hypoxia/acidosis can result. Although position changes may help in the instance of partial or occult prolapse, an overt prolapse requires emergent intervention. Prolonged or persistent cord compression can result in fetal bradycardia with minimal or absent variability. This type of FHR pattern may precede death in utero and is therefore extremely concerning (
Figure 3-7).
Although decreased oxygenation of the fetus is associated with acute episodes of bradycardia (as may result from transitory events of tachysystole or maternal hypotension), variability will usually remain present, provided such occurrences are short-lived. Once the event is corrected and fetal oxygenation resumes, the FHR will usually recover to its prior baseline rate. Chronically decreased blood flow across the uteroplacental unit is also likely to result in FHR bradycardia. This ongoing instance of hypoxia is more concerning however, as demonstrated by minimal or absent variability of the FHR baseline and the inability to recover with interventions (
Figure 3-8).
Bradycardia is the most common FHR change when uterine rupture occurs during a trial of labor after a previous cesarean birth. Before the onset of the bradycardia, FHR patterns have been reported to range from reassuring to those with variable or late decelerations and fetal tachycardia.
12,
13 This type of bradycardia is representative of fetal hypoxia and requires immediate intervention. In Leung’s study, it was noted that fetal intolerance was the
most common signal and that significant neonatal morbidity occurred if a fetal bradycardia lasted 18 minutes or longer before surgical intervention.
12 The rate of perinatal asphyxia after uterine rupture is reported to be 5.1%.
14If a trial of labor is attempted after a previous cesarean birth, the onset of bradycardia should be seen as a red flag until proven otherwise. Although it is possible for bradycardia to result from other causes (such as those discussed previously in this chapter), the consequences of uterine rupture are severe enough to warrant preparation for operative delivery if the bradycardia cannot be promptly reversed. In the event that the FHR does not recover with primary interventions (maternal position change, administration of intravenous fluids and oxygen, discontinuation of oxytocin, or removal of uterotonic agents and/or the administration of tocolytics
2,
15), expeditious cesarean delivery is indicated (
Figure 3-9).
Nonhypoxic Etiology
If the bradycardia is due to a nonhypoxic cause, such as vagal stimulation during the second stage of labor,
10 it is possible that the fetus can recover. In the second stage of labor, intense intracranial pressure occurs as the fetal head descends through the maternal pelvis and vagina. A second-stage bradycardia may be seen in the adequately oxygenated fetus as a normal response to stimulation of the vagus nerve. The key factor in recognizing the difference between hypoxic and nonhypoxic bradycardia is FHR baseline variability. In hypoxic cases of bradycardia, FHR baseline variability is minimal or absent, whereas, in nonhypoxic instances, it will usually remain moderate (
Table 3-4).